8.2
Covalent Bonding
& Lewis Structures
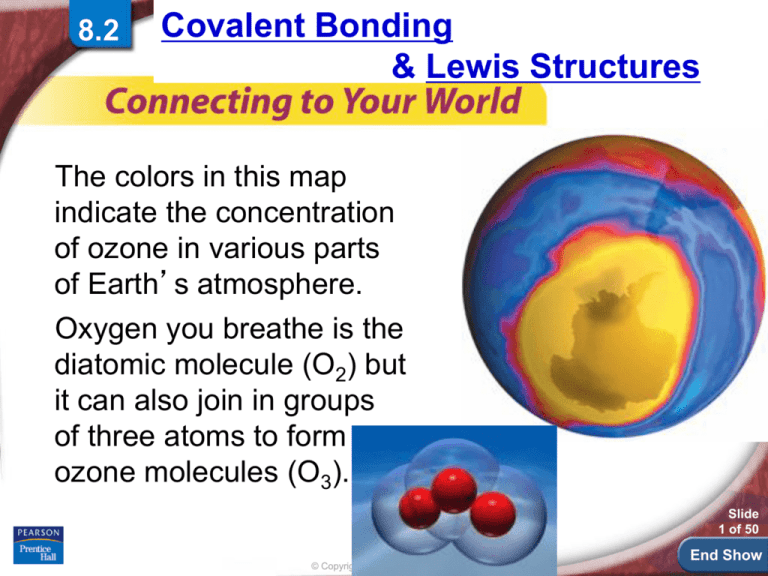
The colors in this map
indicate the concentration
of ozone in various parts
of Earth’s atmosphere.
Oxygen you breathe is the
diatomic molecule (O2) but
it can also join in groups
of three atoms to form
ozone molecules (O3).
Slide
1 of 50
© Copyright Pearson Prentice Hall
End Show
8.2
The Nature of
Covalent Bonding
>
Single Covalent Bonds
Octet Rule:
atoms tend to combine to have 8 electrons in
their outer shell like noble gases.
Slide
2 of 50
© Copyright Pearson Prentice Hall
End Show
8.2
loneofpair
The Nature
Covalent Bonding
> unshared pair
nonbonded pair
1 shared pair
single bond: atoms
bonded by sharing
a pair of electrons.
© Copyright Pearson Prentice Hall
Slide
3 of 50
End Show
8.2
The Nature of
Covalent Bonding
>
structural formula:
shows the arrangement of atoms
How many unshared pairs?
Slide
4 of 50
© Copyright Pearson Prentice Hall
End Show
8.2
The Nature of
Covalent Bonding
>
Single Covalent Bonds
How many shared pairs?
Slide
5 of 50
© Copyright Pearson Prentice Hall
End Show
8.2
The Nature of
Covalent Bonding
>
Double and Triple Covalent Bonds
Double and Triple Covalent Bonds
double bond: bond that shares two pairs
of electrons.
O
O
triple bond: bond that shares three pairs
of electrons.
N
N
Slide
6 of 50
© Copyright Pearson Prentice Hall
End Show
8.2
The Nature of
Covalent Bonding
>
Super Special Carbon
Carbon can form long chains
because it forms up to 4 bonds
on each carbon atom.
Without this property, large
biomolecules could not form
such as:
proteins
lipids (fats)
carbohydrates (sugars/starches)
nucleic acids (DNA/RNA)
© Copyright Pearson Prentice Hall
Slide
7 of 50
End Show
Writing Lewis Structures
PCl3
1. Add valence electrons
of all atoms.
5 + 3(7) = 26
Slide
8 of 50
© Copyright Pearson Prentice Hall
End Show
Writing Lewis Structures
2. Connect all
atoms with single
bonds
Keep track of the electrons:
26 6 = 20
Slide
9 of 50
© Copyright Pearson Prentice Hall
End Show
Writing Lewis Structures
3. Fill the octets of
the outer atoms.
Keep track of the electrons:
26 6 = 20 18 = 2
© Copyright Pearson Prentice Hall
Slide
10 of 50
End Show
Writing Lewis Structures
4. Fill the octet of
the central atom.
Keep track of the electrons:
26 6 = 20 18 = 2 2 = 0
© Copyright Pearson Prentice Hall
Slide
11 of 50
End Show
Writing Lewis Structures
5. If you run out of
electrons before the
central atom has an
octet…
…form multiple
bonds until it does.
Slide
12 of 50
© Copyright Pearson Prentice Hall
End Show
8.1
Section Assessment
Draw the Lewis structure for formaldehyde,
CH2O
Draw the Lewis structure for phosphate, PO4-3
Slide
13 of 50
© Copyright Pearson Prentice Hall
End Show
Quick Quiz!
1. In covalent bonding, atoms attain an octet
electron configuration like noble gases by
A. losing electrons.
B. gaining electrons.
C. transferring electrons.
D. sharing electrons.
Slide
14 of 50
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
2. Electron dot diagrams are superior to
molecular formulas in that they
A. show which electrons are shared.
B. indicate the number of each kind of atom in
the molecule. molecular formulas
C. show the arrangement of atoms in the
molecule. structural formulas
D. are easier to write or draw.
Slide
15 of 50
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
3. Carbon atoms have 4 valence electrons to
form up to 4 bonds. This allows carbon to
form _________________ needed for life.
A. large, long chain biomolecules.
B. nucleic acids
C. carbohydrates
D. all of the above
Slide
16 of 50
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
4. Which of the following diatomic molecules
have a triple bond?
(hint: draw the Lewis structures)
A. O2
O
O
B. N2
N
N
C. Br2
Br
Br
D. H2
H
H
Slide
17 of 50
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
5. Draw the correct Lewis structure for nitrous
(or laughing gas), N2O.
hint: arrange the atoms as…
N
N O
N
N O
Slide
18 of 50
© Copyright Pearson Prentice Hall
End Show








