introduction
advertisement

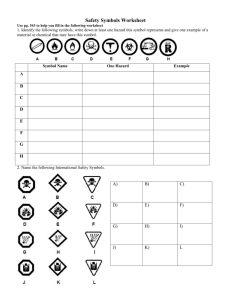
Chemistry in the Community INTRODUCTION Room A-34 Period:__________ Name:___________________ 1 Name:_____________________ Date:______ Period:____ Date Due:_____ ChemCom - Know Your Textbook Please complete this assignment carefully, neatly, and accurately answering all questions. 1. Using the Contents section, how many Units is this text divided into? How many Sections are in each Unit? 2. What is at the end of each Section? 3. What Scenario is investigated in Unit 3? (Hint: see preface xxi) 4. What activity is at the end of each Unit? 5. Look at page 137. When you are reading the book, how do you recognize a term as a key word? Give the key word on page 137 and its definition. 2 6. Locate the Glossary and define the word ion. 7. Describe the picture in Figure 2.11. 8. On what page can you find a picture of a precipitate? What color is the precipitate in this picture? 9. Where is a Periodic Table of the Elements conveniently located? 3 Name:_____________________ Date:______ Period:____ Date Due:_____ Elements and Symbols to Learn As a student of chemistry, you must eventually become familiar with the names and symbols of many elements. Listed below are elements that you must be able to recognize. If given a symbol, you must be able to spell the name of the element. If given the name of an element, you must be able to provide the chemical symbol. Using your book, fill in the symbol of the element next to the name of that element. You must also know the spellings of these elements as well. Elements that are in bold face type are those that do not readily correspond with symbols of similar lettering. Aluminum Antimony Argon Arsenic Barium Beryllium Bismuth Boron Bromine Cadmium Calcium Carbon Chlorine Chromium Cobalt Copper Fluorine Gold Helium Hydrogen Iodine Iron Krypton Lead Lithium Magnesium Manganese Mercury Neon Nickel Nitrogen Oxygen Phosphorus Platinum Potassium Radon Selenium Silicon Silver Sodium Strontium Sulfur Tin Titanium Uranium Xenon Zinc 4 5 6 Name:_____________________ Date:______ Period:____ Date Due:_____ What Has Chemistry Given Us Worksheet 1. List ten things that chemistry has given us. __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ 2. What does soluble mean? 3. Write the name of the elements and their symbols in calcium fluorophosphate? 4. Define Chemistry. 5. Draw benzene based on the description given in the article. 6. Why is it important to know how a molecule is put together? 7. Who is Dmitry Mendeleyev and what did he do? 8. What did Joseph Priestley discover? 9. What did Marie Curie find as the source of radiation in pitchblende? 10. Define polymer. 7 CHEMISTRY LAB EQUIPMENT 8 9 Lecture Notes - Units of Measurement Measurement – _______________________________________________________________________ The problem is, what do you use as a standard? Standard should be an _______________________________________________________________________________ ____________________________________________________________________________. The SI System Important base units to know: Quantity Unit Mass gram Length meter Time second Temperature kelvin Amount of substance mole Abbreviation Important prefixes(multiples of base units) to know: Prefix Abbreviation Meaning Example 10 Reading graduated cylinders: Scientific Notation: 30 300 3000 0.3 0.03 0.003 Factor Label Method (Dimensional Analysis) A method of problem solving that treats units like algebraic factors Rules 1. Put the known quantity over the number 1. 2. On the bottom of the next term, put the unit on top of the previous term. 3. On top of the current term put a unit that you are trying to get to. 4. On the top and bottom of the current term, put in numbers in order to create equality. 5. If the unit on top is the unit of your final answer, multiply/divide and cancel units. If not, return to step # 2. 6. As far as sig figs are concerned, end with what you start with! 11 Example: convert 3.0 ft to inches Example: convert 1.8 years to seconds Example: convert 2.50 ft to cm if 1 inch = 2.54 cm Example: convert 75 cm to m Example: convert 150 g to kg Example: convert 0.75 L to cm3 . Example: convert 22 cm to dm 12 Name:_____________________ Date:______ Period:____ Date Due:_____ Dimensional Analysis Directions: Please solve the following problems for the correct answer and unit using the factor label method. All work must be shown in order to receive full credit. Convert 70 cm to mm Convert 49 cL to dL Convert 6 km to m Convert 142 km to m Convert 47 mL to cL Convert 97 cm to dm Convert 943 g to cg Convert 43 mL to cm3 Convert 75 g to mg Convert 60 km to dm Convert 109 g to kg Convert 13 kL to dL Convert 70 cm3 to mL Convert 55 L to kL Convert 536 km to mm Convert 176 m to mm Convert 250 m to dm Convert 20 mm to dm Convert 2.44 L to cm3 Convert 643 kg to cg Convert 45654 mg to kg Convert 2570 mm to dm Convert 1000 mg to g Convert 160 dm to cm 13