Chapter 10 ppt
advertisement

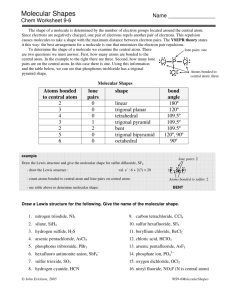
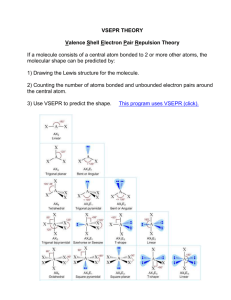
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 1 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear B B 2 0 lone pairs on central atom Cl Be Cl 2 atoms bonded to central atom 3 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear 0 trigonal planar trigonal planar AB3 3 4 Boron Trifluoride 5 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear trigonal planar tetrahedral AB3 3 0 trigonal planar AB4 4 0 tetrahedral 6 Methane 7 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear trigonal planar AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral AB5 5 0 trigonal bipyramidal trigonal bipyramidal 8 Phosphorus Pentachloride 9 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear trigonal planar AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral AB5 5 0 trigonal bipyramidal trigonal bipyramidal AB6 6 0 octahedral octahedral 10 Sulfur Hexafluoride 11 12 bonding-pair vs. bonding- lone-pair vs. bondinglone-pair vs. lone-pair < < pair repulsion pair repulsion repulsion 13 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB3 3 0 AB2E 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar trigonal planar trigonal planar bent 14 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 AB3E 3 1 Arrangement of electron pairs Molecular Geometry tetrahedral tetrahedral tetrahedral trigonal pyramidal 15 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 Arrangement of electron pairs Molecular Geometry tetrahedral tetrahedral AB3E 3 1 tetrahedral trigonal pyramidal AB2E2 2 2 tetrahedral bent 16 VSEPR Class AB5 AB4E # of atoms bonded to central atom 5 4 # lone pairs on central atom Arrangement of electron pairs Molecular Geometry 0 trigonal bipyramidal trigonal bipyramidal 1 trigonal bipyramidal distorted tetrahedron 17 VSEPR Class AB5 # of atoms bonded to central atom 5 # lone pairs on central atom 0 AB4E 4 1 AB3E2 3 2 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron T-shaped 18 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 AB4E 4 1 AB3E2 3 2 AB2E3 2 3 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron trigonal bipyramidal linear T-shaped 19 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral octahedral AB5E 5 1 octahedral square pyramidal Arrangement of electron pairs Molecular Geometry 20 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral octahedral AB5E 5 1 octahedral AB4E2 4 2 octahedral square pyramidal square planar Arrangement of electron pairs Molecular Geometry 21 22 Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of lone pairs on the central atom and number of atoms bonded to the central atom. 3. Use VSEPR to predict the geometry of the molecule. 23 Example 10.1 Use the VSEPR model to predict the geometry of the following molecules and ions: (a) AsH3 (b) OF2 (c) (d) (e) C2H4 Dipole Moments and Polar Molecules electron poor region electron rich region H F d+ d- m=Qxr Q is the charge r is the distance between charges 1 D = 3.36 x 10-30 C m 25 Behavior of Polar Molecules field off field on 26 Bond moments and resultant dipole moments in NH3 and NF3. 27 28 Example 10.2 Predict whether each of the following molecules has a net dipole moment: (a) BrCl (b) BF3 (trigonal planar) (c) CH2Cl2 (tetrahedral) Change in Potential Energy of Two Hydrogen Atoms as a Function of Their Distance of Separation 30 Change in electron density as two hydrogen atoms approach each other. 31 Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals b. Overlap of hybrid orbitals with other hybrid orbitals 32 Formation of sp Hybrid Orbitals 33 Formation of sp2 Hybrid Orbitals 34 Formation of sp3 Hybrid Orbitals 35 Formation of Covalent Bonds in CH4 36 sp3-Hybridized N Atom in NH3 Predict correct bond angle 37 How do I predict the hybridization of the central atom? 1. Draw the Lewis structure of the molecule. 2. Count the number of lone pairs AND the number of atoms bonded to the central atom # of Lone Pairs + # of Bonded Atoms Hybridization Examples 2 sp BeCl2 3 sp2 BF3 4 sp3 CH4, NH3, H2O 5 sp3d PCl5 6 sp3d2 SF6 38 39 Example 10.3 Determine the hybridization state of the central (underlined) atom in each of the following molecules: (a) BeH2 (b) AlI3 (c) PF3 Describe the hybridization process and determine the molecular geometry in each case. sp2 Hybridization of Carbon 41 Unhybridized 2pz orbital (gray), which is perpendicular to the plane of the hybrid (green) orbitals. 42 Bonding in Ethylene, C2H4 Sigma bond (s) – electron density between the 2 atoms Pi bond (p) – electron density above and below plane of nuclei of the bonding atoms 43 Sigma (s) and Pi Bonds (p) Single bond 1 sigma bond Double bond 1 sigma bond and 1 pi bond Triple bond 1 sigma bond and 2 pi bonds 44 Another View of p Bonding in Ethylene, C2H4 45 sp Hybridization of Carbon 46 Bonding in Acetylene, C2H2 47 Another View of the Bonding in Acetylene, C2H2 48 Example 10.5 Describe the bonding in the formaldehyde molecule whose Lewis structure is Assume that the O atom is sp2-hybridized. Experiments show O2 is paramagnetic O O No unpaired eShould be diamagnetic Molecular orbital theory – bonds are formed from interaction of atomic orbitals to form molecular 50 orbitals. Energy levels of bonding and antibonding molecular orbitals in hydrogen (H2). A bonding molecular orbital has lower energy and greater stability than the atomic orbitals from which it was formed. An antibonding molecular orbital has higher energy and lower stability than the atomic orbitals from which it was 51 formed. General molecular orbital energy level diagram for the second-period homonuclear diatomic molecules Li2, Be2, B2, C2, and N2. 52 Delocalized molecular orbitals are not confined between two adjacent bonding atoms, but actually extend over three or more atoms. Example: Benzene, C6H6 Delocalized p orbitals 53 Bonding in the Carbonate Ion, CO32- 54