Novak - York College of Pennsylvania
advertisement

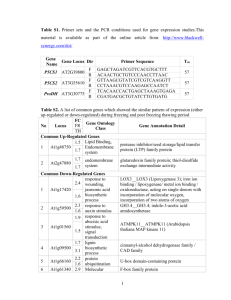
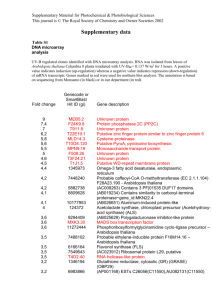
Comparing the Nucellar Growth Index in Megagametogenesis of two ecotypes of Arabidopsis thaliana Heynh in Holl & Heynh Emily Novak, Department of Biology, York College Introduction • Arabidopsis thaliana (L.) Heynh is an essential model in the study of plant sciences, valued for its rapid life cycle and its easy cultivation (Smyth 1990). • Throughout both megasporogenesis and megagametogenesis, the nucellus of the ovule expands with each mitotic division, consuming the surrounding tissue of the hyponucellus and the epinucellus. • The nucellar growth index was proposed in order to observe and determine just how much of the tissue surrounding the nucellus is consumed during these stages of ovule growth (Personal communication, J. M. Herr). Figure 4. Nucellar growth indexes of both A. thaliana ecotypes, Landsberg erecta and Columbia, comparing the functional stage to the 8-nucleate stage of megagametogenesis. Groups are not significantly different as indicated by a paired t-test (two-tailed p = 0.1554). Objective • The objective of this study is to apply an innovative calculation, termed the Nucellar Growth Index, in order to compare two ecotypes, Landsberg erecta and Columbia, of A. thaliana. Figure 1. Nucellar Growth Profile and Nucellar Growth Index original concept as presented by Dr. J. M. Herr (Personal Communication). Methods Whole inflorescences collected and fixed in FPA50 Seeds of Arabidopsis thaliana Landsberg erecta and Columbia planted Results Table 1. Mean Nucellar Growth Indexes of megagametophytic stages in two ecotypes of Arabidopsis thaliana. 24 hours Dehydrated to 100% ethyl alcohol 70% ethyl alcohol 10 minutes in 75%, 80%, 85%, 90%, 95% Herr clearing fluid Dissected using Nikon SMZ 1500 dissecting microscope Photographed with a Nikon DS-Ri1 camera Observed under oil immersion using a Nikon Eclipse 80i phase contrast microscope Functional v 2-nucleate 2-nucleate v 4-nucleate 8-nucleate v 4-nucleate 1mean Nucellar Growth Index1 Columbia Landsberg erecta 1.877 1.320 1.168 1.972 0.909 1.347 Sample sizes were n = 10 for each group and two groups were used to calculate nucellar growth index for each column giving a total sample size of n = 20 for each column (Figures 2-5). Figure 5. Nucellar growth indexes of intermediate stages of megagametogenesis of the Columbia ecotype of A. thaliana. Sample sizes were n = 10 for each group and two groups were used to calculate nucellar growth index for each column giving a total sample size of n = 20 for each column. Groups are significantly different (p = 0.0030) as indicated by a one-way ANOVA test. Conclusions • The tissue making up the epinucellar region is completely consumed by the nucellar region in both ecotypes by the 8nucleate stage. • In both ecotypes of A. thaliana, Landsberg erecta and Columbia appear to have similar overall net growth patterns in regards to the nucellus. • When comparing the intermediate stages of nucellar growth, the ecotypes are in fact significantly different. - Landsberg erecta expresses its greatest net growth of the nucellus during the 2-nucleate to 4-nucleate interval. - Columbia exhibited the greatest net growth of the nucellus during the functional to 2-nucleate interval. Literature Cited Measurements were taken of epinucellus, nucellus, and hyponucellus and applied to the Nucellar Growth Index calculation: eM + L′ − L + hM (eI + hI) Statistical analysis Smyth, David R., John L. Bowman, and Elliot M. Meyerowitz. 1990. Early Flower Development in Arabidopsis. The Plant Cell 2: 755-767 Future Studies Figure 2. Nucellar growth indexes of intermediate stages of megagametogenesis of the Columbia ecotype of A. thaliana. Groups are significantly different (p = 0.0030) as indicated by a oneway ANOVA test. Figure 3. Comparison of the nucellar growth indexes of intermediate stages of Landsberg erecta ecotype of A. thaliana. Groups were found to be significantly different (p = <0.0001) as indicated by a one-way ANOVA test. Since this study was the first documented of its kind, the possibilities for future studies are virtually endless and can provide morphologists with yet another feature in which plants can be observed, measured, and possibly even differentiated from one another. Acknowledgements I would like to thank Dr. Smith for his unyielding patience. I would also like to thank Dr. John Herr for his passion of plant morphology which lead to the contribution of the Nucellar Growth Index as well as his insight throughout my research project.