TPJ_4860_sm_SupplementalFileLegends-1
advertisement

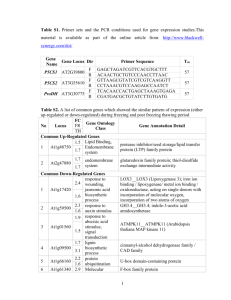
Supplemental Information Legends Supplemental Figure S1. Structure of AGPase proteins from plants and bacteria. The small (SSU) and large (LSU) subunits of the Arabidopsis thaliana AGPase (plastidial) were aligned with the small subunit of the cytosolic isoform from rice endosperm (NP_001061603), and with AGPase proteins from the cyanobacterium Synechocystis sp. PCC6803 (NP_443010) and Escherichia coli (glgC; CAA23544). The arrows indicate the five cysteine residues in the A. thaliana small subunit protein and analogous cysteine residues in the other proteins. Supplemental Figure S2. Arabidopsis thaliana APS1 (At5g48300) genomic region used for site directed mutagenesis and complementation of the adg1 mutant. Exons are shaded in grey, with protein coding regions shown in bold. The translation initiation and stop codons of the APS1 gene are highlighted in green, and the codons encoding the five Cys residues targetted for site-directed mutagenesis are shown in red. The APS1 gene was amplified from A. thaliana Col-0 genomic DNA by PCR using AtAPS1-5 and AtAPS1-3 primers. The annealing site of the AtAPS1-5 primer (underlined) lies within the 3´-UTR of the upstream At5g48290 gene (encoding a heavy metal transporter/detoxification protein), thus the amplified fragment includes the entire 446-bp intergenic region upstream of the APS1 gene, which is presumed to contain the promoter region of the APS1 gene. The annealing site of the AtAPS1-3 primer (underlined) lies 9-34 bp downstream of the poly-adenylation site of the APS1 pre-mRNA. The AtAPS1-3 primer included an Afe1 restriction site (AGC/GCT) at the 5´-end to enable subsequent blunt-end cloning of the APS1 gene. Supplemental Figure S3. Southern hybridisation analysis of the adg1/APS1C81S and adg1/APS1WT mutants. (A) Schematic overview of the T-DNA region containing the APS1 and bar (BASTA resistance) genes, flanked by the right (RB) and left (LB) borders. Restriction sites within the T-DNA insert used for digestion of genomic DNA are shown, along with the region that hybridizes to the bar probe (red). The expected sizes of hybridising fragments are illustrated below. (B) Autoradiographs of (1) BspLU11I/NdeI (2) SacI and (3) and HindIII restricted genomic DNA (approx. 5 µg) from the T 1-T3 generations of adg1/APS1C81S and adg1/APS1WT mutants, and wild type A. thaliana Col-0 control plants, hybridized with a probe for the bar gene. The molecular size markers (M) were 2, 2.5, 3, 4, 5, 6, 8 and 10 kbp. Arrows mark the 5-kbp band which is close to the expected size of the T-DNA insert (4.57 kbp). Figure S4. Purification of (A) wild type and (B) mutated C81S ADP-glucose pyrophosphorylase from Arabidopsis thaliana leaves. Fractions from successive steps in the purification of AGPase were analysed by SDS-PAGE and staining with Coomassie Blue R250. The samples and amounts of protein loaded in each lane were as follows: (M) Molecular weight markers (BenchMark Protein Ladder, Invitrogen); (1) crude leaf extract, 50 μg; (2) Orange 1 pass through, 45 μg; (3) Orange1 wash, 40 μg; (4) Orange 1 120 mM NaCl wash, 5 μg; (5) Orange1 1 mM 3PGA elution, 1.5 μg; (6) Centricon 100 concentrator filtrate, (50 µla); (7) Centricon 100 concentrator retentate, 30 μg; (8) Superdex 200, 12 μg; (9) MonoQ, (100 µla). aprotein content below the detection limit of dye-binding protein assay, sample contained proteins from the volume indicated precipitated by addition of 4 vols acetone. Protein bands corresponding to the expected size of the small (50 kDa) and large (57 kDa) subunits of AGPase are indicated by arrows. Supplemental Figure S5. Effect of dithiothreitol treatment on the activity of Arabidopsis thaliana AGPase. AGPase was purified from (A) wild type Col-0 and (B) the adg1/APS81S mutant, and the purified enzymes were uncubated with 10 mM reduced DTT (DTTred) or10 mM oxidised DTT (DTTox). AGPase activity was measured in the direction of ADPGlc pyrophosphorolysis, in the absence of the allosteric activator 3PGA. Data are expressed as mean ± SD of three independent measurements. Significant differences between the DTT treatments according to Student’s t-test are indicated by an asterisk (P<0.005). Supplemental Table S1. Purification of ADP-glucose pyrophosphorylase (AGPase) from Arabidopsis thaliana leaves. Wild type and the mutated C81S form of AGPase were extracted from leaves of wild type A. thaliana Col-0 and the adg1(APS1C81S) mutant, respectively. Both forms of the enzyme were purified by Orange 1 dye affinity chromatography, gel filtration (Superdex 200) and anion exchange chromatography (MonoQ). AGPase activity was measured in the direction of ADPG pyrophosphorolysis, with 1 mM 3PGA included in the reaction. Supplemental Table S2a. Peptide analysis of wild type ADP-glucose pyrophosphorylase from Arabidopsis thaliana. AGPase purified from WT A. thaliana Col-0 leaves was subjected to mass spectrometric peptide analysis after trypsin digestion. The total number of spectra and the number of different individual peptides are shown for each protein identified. AGPase subunits are highlighted in bold. Supplemental Table S2b. Peptide analysis of C81S ADP-glucose pyrophosphorylase from Arabidopsis thaliana. The mutated C81S form of AGPase was purified from leaves of the A. thaliana adg1(APS1C81S) mutant and subjected to mass spectrometric peptide analysis after trypsin digestion. The total number of spectra and the number of different individual peptides are shown for each protein identified. AGPase subunits are highlighted in bold. Supplemental Table S2c. APS and APL subunits identified in purified wild type and C81S ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Purified preparations of wild type and the mutated C81S form of A. thaliana leaf AGPase were subjected to mass spectrometric peptide analysis. For each preparation, peptide analysis was also performed on the 57-kDa protein band excised from SDS polyacrylamide gels. The table shows the number of different peptides identified from the various small (APS) and large (APL)subunits of AGPase in both the non-fractionated preparation (Total) and in the 57-kDa fraction. Supplemental Table S3. Oligonucleotides used for site-directed mutagenesis. Point mutations were introduced into the coding sequence of the APS1 gene to change individual cysteine codons (TGT or TGC) to serine codons (TCT or TCA) using the QuickChange® Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. The forward and reverse primers used to mutate the individual cysteine codons are shown, with the introduced serine codons underlined. Supplemental Methods. Purification of Arabidopsis thaliana WT and C81S ADP-glucose pyrophosphorylase (AGPase). Analysis of purified AGPase.