Chapter 24 HEIN

Carboxylic Acids and Esters
Chapter 24
Version 1.0
Hein * Best * Pattison * Arena
Colleen Kelley
Chemistry Department
1
© John Wiley and Sons, Inc.
Chapter Outline
24.1 Carboxylic Acids
24.2
Nomenclature and
Sources of Aliphatic
Carboxylic Acids
24.3
Physical Properties of
Carboxylic Acids
24.4
Classification of Carboxylic
Acids
24.5
Preparation of Carboxylic
Acids
2
Chapter Outline (continued)
24.6
Chemical Properties of
Carboxylic Acids
24.7
Nomenclature of Esters
24.8
Occurrence and
Physical Properties of
Esters
24. 9 Polyesters:
Condensation
Polymers
24.10
Chemical Properties of
Esters
24.11
Glycerol Esters
24.12
Soaps and Synthetic
Detergents
24.13
Esters and Anhydrides of Phosphoric Acid
3
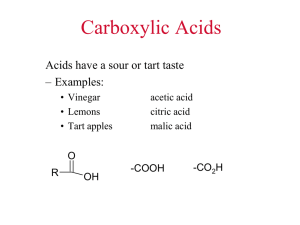
Carboxylic Acids
4
• The functional group of the carboxylic acid is called a carboxyl group and is represented in the following ways:
O
OH or
-COOH or
CO
2
H
5
Nomenclature and
Sources of
Aliphatic
Carboxylic Acids
6
IUPAC Rules for Naming Carboxylic Acids
1. To establish the parent name, identify the longest carbon chain that includes the carboxyl group.
2. Drop the final
–e from the corresponding hydrocarbon name.
3. Add the suffix
–oic acid
.
HCOOH, methan oic acid
CH
3
COOH ethan oic acid
CH
3
CH
2
COOH propan oic acid
7
Naming Carboxylic Acids
• Other groups bonded to the parent chain are numbered and named as we have done previously.
5
CH
3
4
CH
2
3 2
CHCH
2
1
COOH
CH
3
3-methylpentanoic acid
8
9
Nomenclature of Carboxylic Acids
• Use of Greek letters:
5
CH
3
4
CH
3
2
CH
2
2
1
2
COOH
CH
3
CH
2
CHCOOH
OH
-hydroxybutyric acid
2-hydroxybutanoic acid
10
Physical Properties of
Carboxylic Acids
11
Physical Properties of Carboxylic Acids
• Each aliphatic carboxylic acid molecule is polar and consists of a carboxylic acid group and a hydrocarbon group (-R).
– Carbons 1-4 = water soluble
– Carbons 5-8 = slightly water soluble
– Carbons 8 and above = virtually insoluble in water
12
Physical Properties of Carboxylic Acids
• The comparatively high boiling points for carboxylic acids are due to intermolecular attractions resulting from hydrogen bonding .
O HO
R C C R
OH O
13
Physical Properties of Carboxylic Acids
• Carboxylic acids are generally weak acids; that is, they are only slightly ionized in water.
O
+ H
C
H
3
C acetic acid
OH
2
O
O hydronium ion
+ H
3
O
+
C
H
3
C acetate ion
O
-
14
Classification of
Carboxylic Acids
15
Classification of Carboxylic Acids
• saturated monocarboxylic acids
• unsaturated carboxylic acids
• aromatic carboxylic acids
• dicarboxylic acids
• hydroxy acids
• amino acids
16
Unsaturated Carboxylic Acids
• An unsaturated acid contains one or more C=C.
– Acrylic acid, CH
2
=CHCOOH, also called propenoic acid.
• Even one C=C bond exerts an influence on the physical and chemical properties of the acid.
Ex: stearic acid CH
3
(CH
2
)
16
COOH, mp = 70 C vs.
oleic acid CH
3
(CH
2
)
7
CH=CH(CH
2
)
7
COOH, mp = 16 C
17
Aromatic Carboxylic Acids
• In an aromatic carboxylic acid, the carbon of the carboxyl group (-COOH) is bonded directly to a carbon in an aromatic ring.
COOH
COOH benzoic acid o-toluic acid
CH
3
18
19
Hydroxy Acids
• Hydroxy acids have the functional group of an alcohol and a carboxylic acid.
COOH
CH
3
CHCOOH
OH
2-hydroxypropanoic acid lactic acid
OH
o-hydroxybenzoic acid salicylic acid
20
Amino Acids
• Each amino acid molecule has a carboxyl group that acts as an acid and an amino group that acts as a base.
• About 20 biologically important amino acids, each with a different group represented by R, are found in nature.
NH
2
CHCOOH
R
21
Preparation of
Carboxylic Acids
22
Preparation of Carboxylic Acids
• oxidation of an aldehyde or primary alcohol
• oxidation of alkyl groups attached to aromatic rings
• hydrolysis of nitriles
23
Oxidation of an Aldehyde or a
Primary Alcohol
H O
R C OH
[O]
R
H primary (1 o
) alcohol
C H + H
[O] = Cr
2
O
7
2-
2
O
[O]
R
O
C OH
24
Oxidation of Alkyl Groups
Attached to Aromatic Rings
CH
3
COO
-
Na
+ toluene
CH
2
CH
3
NaMnO
4
NaOH heat sodium benzoate
COO
-
Na
+ ethylbenzene
NaMnO
4
NaOH heat sodium benzoate
+ CO
2
(g)
25
Hydrolysis of Nitriles
• RCN + 2 H
2
O
+
RCOOH + NH
4
+
26
Chemical Properties of
Carboxylic Acids
27
Chemical Properties of
Carboxylic Acids
1. Acid-Base reactions
2. Substitution reactions
• acid chlorides
• acid anhydrides
• esters
• amides
28
Acid-Base Reactions
•
Because of their ability to form hydrogen ions in solution, acids in general have the following properties:
1.
Sour taste
2.
Change blue litmus to red and affect other suitable indicators.
3.
Form water solutions with pH values less than 7.
4.
Undergo neutralization reactions with bases for form water and a salt.
29
H
3
C
Acidity of Carboxylic Acids
O O
C
O H + H
2
O H
3
C
C
O
+ H
3
O
+
O O
H
3
C
C
O H + NaOH H
3
C
C
O
-
Na
+ + H
2
O
30
Substitution Reactions
• acid chlorides
• acid anhydrides
• esters
• amides
31
Acid Chloride Formation
•
Thionyl chloride (SOCl
2
) reacts with carboxylic acids to form acid chlorides.
O
C
R OH carboxylic acid
+ SOCl
2 thionyl chloride
R
O
C
Cl acid chloride
+ SO
2
+ HCl
32
Acid Anhydride Formation
•An organic anhydride is formed by the elimination of water from two molecules of carboxylic acid.
R
O
C
O H
+
HO
O
C
R' R
O O
C C
O anhydride
R'
+ H
2
O
33
Ester Formation
• An ester is formed by the reaction of an acid with an alcohol or a phenol; water is also produced in the reaction:
O
R
C carboxylic acid
OH
+
R'
H O alcohol
H
+
R
O
C ester
O
R'
+ H
2
O
34
Nomenclature of
Esters
35
Nomenclature of Esters
• The alcohol part is named first, followed by the name of the acid modified to end in
–ate
.
O
O
C R'
R acid
O alcohol
C
H
3
C ethanoate or acetate
O
CH
3 methyl methyl ethanoate or methyl acetate
36
37
Occurrence and
Physical Properties of Esters
38
Properties of Esters
• Simple esters derived from monocarboxylic acids and monohydroxy alcohols are colorless, generally nonpolar liquids or solids.
• Low- and intermediate-molar-mass esters
(both acids and alcohols up to about 10 carbons) are liquid with characteristic
(usually fragrant or fruity) odors.
39
Occurrence and Properties of Esters
• High-molar-mass esters (formed from acids or alcohols of 16 or more carbons) are waxes and are obtained from various plants.
– They are used in furniture wax and automobile wax preparations.
– Carnauba wax contains esters of 24-and 28carbon fatty acids and 32- and 34-carbon alcohols.
40
Polyesters:
Condensation Polymers
41
Polyesters: Condensation Polymers
• Condensation polymers are formed by substitution reactions between neighboring monomers.
• The polyesters are joined by ester linkages between carboxylic acid and alcohol groups.
– The macromolecule formed may be linear or cross-linked.
42
Polyesters: Condensation Polymers diacid diol
HOOC(CH
2
) x
COOH + HO(CH
2
) y
OH polyester
-C(CH
2
) x
C-O(CH
2
) y
O-
O O
43
Chemical
Properties of Esters
44
Hydrolysis
• The most important reaction of esters is hydrolysis – the splitting of molecules through the addition of water.
• A catalyst is often required.
– An acid or base
– In living systems, enzymes act as catalysts.
45
Acid Hydrolysis
R'
• The hydrolysis of an ester involves the reaction with water to form a carboxylic acid and an alcohol.
O
O
C
O ester
H
+
R
+ H
2
O or enzyme
R'
O H alcohol
+
HO
C carboxylic acid
R
46
Alkaline Hydrolysis (Saponification)
•
Saponification is the hydrolysis of an ester by a strong base (NaOH or KOH) to produce an alcohol and a salt (or soap if the salt formed is from a high-molar-mass acid).
• Notice that in saponification, the base is a reactant and not a catalyst.
O
O
R' C
O ester
R
+ NaOH
H
2
O
R'
OH alcohol
+
R
C salt
O
-
Na
+
47
Glycerol Esters
48
Fats and Oils
• Fats and oils are esters of glycerol and predominantly long-chain fatty acids.
• Fats and oils are also called triacylglycerols or triglycerides , since each molecule is derived from one molecule of glycerol and three molecules of fatty acid:
49
glycerol portion
H
H
H
C
C
H C
O
O
O
O
O
C R
C
O
R'
C R"
H
General formula for a triacylglycerol
50
Triacylglycerol
The structural formulas of triacylglycerol molecules differ because:
1.
The length of the fatty acid chain varies from 4 to 20 carbons, but the number of carbon atoms in the chain is nearly always even.
2.
Each fatty acid may be saturated or unsaturated and may contain one, two, or three C=C.
3.
A triacylglycerol may, and frequently does, contain three different fatty acids.
51
52
• The most abundant unsaturated acids in fats and oils contain 18 carbon atoms.
• In all of these naturally occurring unsaturated acids, the configuration about C=C is cis .
53
Physical Differences Between Fats & Oils
• Fats are solid; oils are liquid at room temperature
• Fats contain a larger portion of saturated fatty acids whereas oils contain greater amounts of unsaturated fatty acids.
– Polyunsaturated means that each molecule of fat contains several C=C.
54
Comparison of Fats & Oils
• Fats come from animal sources:
–Lard from hogs, tallow from cattle and sheep
• Oils come from vegetable sources:
–Olives, corn, peanut, soybean, canola
55
Hydrogenation of Glycerides
• Hydrogen adds to the C=C of oil to saturate it and form fats:
• H
2
+ -CH=CH-
-CH
2
-CH
2
-
• In practice, only some of the C=C are allowed to become saturated.
– Partial hydrogenation
56
Hydrogenolysis
• Triacylglycerols can be split and reduced in a reaction called hydrogenolysis
(splitting by hydrogen).
57
Hydrolysis
• Triacylglycerols can be hydrolyzed , yielding fatty acids and glycerol.
58
Saponification
• The saponification of a fat or oil involves the alkaline hydrolysis of a triester.
• The products formed are glycerol and the alkali metal salts of fatty acids, which are called soaps.
59
Soaps and Synthetic
Detergents
60
Soaps and Synthetic Detergents
• In the broadest sense possible, a detergent is simply a cleansing agent.
• A soap is distinguished from a synthetic detergent on the basis of chemical composition and not on the basis of function or usage.
61
Soaps
• Salts of long-chained fatty acids are called soaps.
• Fat or oil + NaOH
Soap + Glycerol
62
Figure 24. 1 Cleansing action of soap.
63
Synthetic Detergents - Anionic
The one great advantage these synthetic detergents have over soap is that their Ca +2 , Mg +2 , and Fe +3 salts, as well as their Na +1 salts, are soluble in water. Therefore, they are nearly as effective in hard water as in soft water.
sodium lauryl sulfate
OSO
3
-
Na
+ nonpolar hydrophobic end, grease soluble polar hydrophilic end, water soluble
64
Synthetic Detergents – Nonionic
• The molecule of a nonionic detergent contains a grease-soluble component and a water soluble component.
• Some of these substances are especially useful in automatic washing machines because they have good detergent, but low sudsing, properties.
CH
3
(CH
2
)
10
CH
2
-O-(CH
2
CH
2
O)
7
-CH
2
CH
2
OH grease soluble, hydrophobic water soluble, hydrophilic
65
Biodegradability
• Organic substances that are readily decomposed by microorganisms in the environment are said to be biodegradable.
• Detergents that contain straight-chain alkyl groups are biodegradable.
66
a biodegradable detergent
OSO
3
-
Na
+ a nonbiodegradable detergent
OSO
3
-
Na
+
67
Esters and
Anhydrides of
Phosphoric Acid
68
Phosphoric Acid
• Phosphoric acid has a Lewis structure similar to that of a carboxylic acid.
• Phosphoric acid reacts with an alcohol to form a phosphate ester.
O
O
P
HO
OH phosphoric
OH acid
+ HOCH
2
CH
3 ethanol
P
OCH
2
CH
3
HO
OH monoethyl phosphate
+ H
2
O
69
70