Document
advertisement

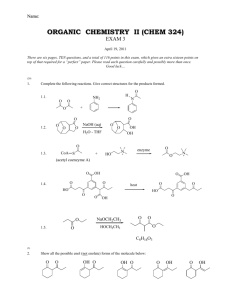
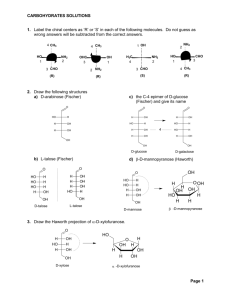
Chapter 17 Carbohydrates (糖,碳水化合物) Ref: Wade, chapter 23 曾昭琼,第十九章 Carbohydrates (糖,碳水化合物) D-glucose (葡萄糖) H HO H H CHO (R) OH (S) H (R) OH (R) OH CH2OH H HO H H CHO OH H OH OH CH2OH D-fructose (果糖) CH2OH C O (S) HO H (R) H OH (R) H OH CH2OH CH2OH C O HO H H OH H OH CH2OH Sugars (糖) They have the molecular formulas Cn(H2O)n Carbohydrates (糖,碳水化合物) Polyhydroxyaldehydes, polyhydroxyketones, and compounds that can be hydrolyzed to them are classified as carbohydrates (多羟基醛、多羟基酮及凡是能水解得 到多羟基醛或多羟基酮的化合物均是碳水化合物) 葡萄糖(glucose)、果糖(fructose)、半乳糖(galactose) 蔗糖(sucrose)、麦芽糖(maltose)、乳糖(lactose) 淀粉(starch)、纤维素(cellulose) Classification of carbohydrates monosacharides (单糖)★: simple sugar 葡萄糖(glucose)、果糖(fructose)、半乳糖(galactose) disaccharides (二糖) 蔗糖(sucrose)、麦芽糖(maltose)、乳糖(lactose) oligosaccharides (寡糖) polysaccharides (多糖) 淀粉(starch)、纤维素(cellulose) Monosaccharides 单糖 1. Classification of Monosaccharides Polyhydroxy aldehydes are aldoses (醛糖) Polyhydroxy ketones are ketoses (酮糖) trioses 三碳糖,丙糖 tetroses 四碳糖,丁糖 pentoses 五碳糖,戊糖 hexoses 六碳糖,己糖 heptoses 七碳糖,庚糖 aldohexose 己醛糖 ketohexose 己酮糖 D/L: D and L notations are used to describe the configurations of carbohydrates (根据构型分类) CHO CHO H OH CH2OH D-(+)-Glyceraldehyde D-(+)-¸ÊÓÍÈ© H HO H H CHO OH H OH OH CH2OH D-glucose D-葡萄糖 HO H CH2OH L-(-)-Glyceraldehyde L-(-)-¸ÊÓÍÈ© HO H HO HO CHO H OH H H CH2OH L-glucose L-葡萄糖 2. Structures of monosaccharides Relative configuration 相对构型 D- /L- : erythreo- /threo- 赤式/苏式 Absolute configuration 绝对构型 R- /S- Open-chain structure 开链结构 Cyclic structure 环状结构 ref: 图19-1 or figure 23-3 Family tree of D-aldoses D-甘油醛 D-苏阿糖 D-赤藓糖 D-核糖 D-葡萄糖 D-树胶糖 阿拉伯糖 D-甘露糖 D-木糖 D-半乳糖 D-异木糖 erythreo赤式 Diastereomer 非对映体 threo苏式 epimers (差向异构体) 具有三个或三个以上手性中心的化合物,若只有 一个手性中心不同,其它都相同,则互称为差向异 构体(epimers) Cyclic Structures of Monosaccharides ----Hemiacetal(半缩醛) Formation CHO H CH2OH O H H OH H H HO OH OH H OH Haworth formulas OH HO HO OH H H H OH H OH CH2OH CH2OH O H H OH H OH H OH OH α-D-(+)-glucopyranose OH Haworth formulas Anomeric carbon 异头碳,端基碳 O OH HO HO OH O OH OH β-D - (+)-glucopyranose anomer 端基(差向)异构体 D-葡萄糖(glucose) 1 CHO 2 H C OH 3 HO C H 4 H 5C OH H C OH 6 CH2OH HOH2C HO HO O OH OH 除1-位碳外,其它 碳上的羟基及羟甲 基均处在平伏键上。 Drawing cyclic monosaccharides 1 CHO 2 H C OH 3 HO C H 4 H 5C OH H C OH 6 CH2OH 6CH2OH H O 5 H H C 4 H OH 1 OH 3 2 * OH H OH ¦Á-D-(+)-Glucopyranose (¦Á-D-(+)-ßÁà«(ÐÍ)ÆÏÌÑÌÇ) H 6 CH2OH 5 OH OH OH 3 H H 4 H 4 2 OH 6CH2OH O 5 H H OH OH 3 H D-葡萄糖 (glucose) CHO 1 H H CH 1 6CH2OH O 2 OH Open-chain form of D-glucose (¿ªÁ´ÐÍD-ÆÏÌÑÌÇ£© 5 H OH OH 3 H H 4 * OH O H 2 OH C 1 H ¦Â-D-(+)-Glucopyranose (¦Â-D-(+)-ßÁà«(ÐÍ)ÆÏÌÑÌÇ) 把羰基碳放在最右侧,把环中氧原子放在右后方。 6CH2OH H O 5 H H C 4 H OH 1 OH 3 2 * OH H OH HOH2C O HO HO OH OH trans-, αα-D-(+)-glucopyranose H 4 6CH2OH O 5 H H OH OH 3 H * OH C 1 2 OH H HOH2C O HO HO OH OH cis-, β β-D - (+)-glucopyranose b-D-glucose is the predominant form at equilibrium D-glucopyranose D-吡喃葡萄糖 1 CHO 2 H C OH 3 HO C H 4 H 5C OH H C OH 6 CH2OH α-D-glucopyranose α-D-吡喃葡萄糖 β-D-glucopyranose β-D-吡喃葡萄糖 D-ribofuranose D-呋喃核糖 α-D-ribofuranose α-D-呋喃核糖 β -D-ribofuranose β -D-呋喃核糖 Note … • If an aldose can form a five- or six-membered ring, it will exist predominantly as a cyclic hemiacetal • Six-membered rings are called pyranoses • Five-membered rings are called furanoses Haworth projections allow us to see the relative orientation of the OH groups in the ring. b- sugar is the predominant form at equilibrium. D-fructose(果糖) mutarotation (变旋现象):a property of anomers CHO OH H H2C HO HO O OH OH ¦Á-D-(+)-Glucopyranose (¦Á-D-(+)-ßÁà«(ÐÍ)ÆÏÌÑÌÇ) (mp, 146¡æ [a]D25 = +1120) HO OH OH H H OH H OH H2C HO HO (¿ªÁ´ÐÍD-ÆÏÌÑÌÇ£© OH OH CH2OH Open-chain form of D-glucose O ¦Â-D-(+)-Glucopyranose (¦Â-D-(+)-ßÁà«(ÐÍ)ÆÏÌÑÌÇ ) (mp, 150¡æ [a]D25 = +18.70) At equilibrium, [α]D25 = +52.6° , including α- 36% β- 64% 3. Reactions of monosaccharides -C=O, -OH • (1) Side reactions in base: epimerization; enediol rearrangement • (2) Reduction: NaBH4 ; H2/catalyst, forming alditols (糖醇) • (3) Oxidation: • Bromine water (Br2-H2O); forming aldonic acid (glyconic acid, 糖酸) • HNO3; forming aldaric acid (糖二酸) • Tollens test; Feilling’s reagent; • (4) Formation of glycosides • (5) Etherification • (6) Acylation: ester formation • (7) Reaction with phenylhydrazines: osazones (糖脎) formation • (8) Chain shortening: the Ruff degradation • (9) Chain lengthening: the Kiliani-Fischer synthesis (1) Side reactions in base: --------epimerization; enediol rearrangement (差向异构化);(烯二醇重排) 23-8 epimerization CHO H HO CHO OH OH H H HO H H OH H OH H OH H OH CH2OH CH2OH D-glucose D-葡萄糖 HOH2C HO HO HO HOH2C O OH D-mannose D-甘露糖 OH HO HO OH O OH D-fructose D-果糖 D-glucose D-葡萄糖 enediol rearrangement D-mannose D-甘露糖 (2) Reduction of Monosaccharides 23-9 The carbonyl of aldoses and ketoses can be reduced by the carbonyl-group reducing agents to form alditols(糖醇) CHO HOH2C HO HO H O OH OH HO OH H H NaBH4 OH or H2/Ni H OH CH2OH D-Glucose CH2OH H HO OH H H OH H OH CH2OH D-Glucitol (D-ÆÏÌÑÌÇ´¼£© (3) Oxidation of monosaccharides; reducing sugars 23-10 A) Br2-H2O The aldehyde groups can be oxidized Ketones and alcohols cannot be oxidized by Br2 Br2-H2O can be used to determine aldehydes and ketones B) Nitric acid (HNO3) CH2OH COOH O HO H HNO3 HO H H OH H OH H OH H OH CH2OH COOH C) Tollens test: Ag(NH3)2+ CHO H HO COOH OH H Ag(NH3)2 + H HO OH H H OH H OH H OH H OH CH2OH CH2OH CH2OH COOH O HO H + Ag mirror H Ag(NH3)2+ HO COOH OH H + HO H HO H H OH H OH H OH H OH H OH H OH CH2OH CH2OH CH2OH • Sugars that reduce Tollens reagent to give a silver mirror are called reducing sugar(还原性糖). D) Periodic acid (HIO4) cleavage of sugars H H C OH H + O H C OH H C OH + 2 HIO4 H C OH H + H O H O 1 H HO H H C formaldehyde (¼×È©) O H 1 H C OH + OH OH OH 6 CH2OH formaldehyde (¼×È©) O H H formic acid (¼×Ëᣩ + 5 HIO4 formic acid (¼×Ëᣩ O 4 H C OH H + C O H 6 formic acid (¼×Ëᣩ formaldehyde (¼×È©) (4) Formation of glycosides (糖)苷 The acetal (or ketal) of a sugar is called a glycoside. CHO H HO OH OH H H OH H OH O HO HO OH CH3OH HCl OH CH2OH D-(+)-Glucose OH HO HO OH O + OH OCH3 methyl α-D-glucopyranoside (甲基-α-D-(+)-吡喃葡萄糖苷 ) (mp, 165℃ [α]D25 = +158°) HO HO O OCH3 OH methyl β-D-Glucopyranoside (甲基- β-D-(+)-吡喃葡萄糖苷 ) (mp, 107℃ [α]D25 = -33°) Nonreducing sugars 非还原性糖 Mechanism of Glycoside Formation Formation of an N-Glycoside (5) Etherification 醚化 OH OCH3 CH3__OSO3CH3 O HO HO OH -OH H3CO H3CO OH Methyl 2,3,4,6-tetra-O-methyl-β-Dglucopyranoside OCH3 CHO OCH3 H3O+ O OCH3 H2O OCH3 OCH3 OCH3 Reagents: CH3OSO3CH3-NaOH; CH3I-Ag2O H3CO H3CO O H3CO H3CO H O H3CO OH OCH3 OCH3 H H OCH3 H OH 2,3,4,6-tetra-O-methyl-D-glucose (2,3,4,6-四-O-甲基葡萄糖) CH2OCH3 (6) Acylation: ester formation 五乙酰葡萄糖 Reagents: RCOCl or RCOOCOR, base; (7) Reaction with phenylhydrazines: ------osazones (糖脎) formation osazones (糖脎) HO HO H H CHO H H OH OH CH2OH D-mannose D-甘露糖 3PhNHNH2 HC NNHPh C NNHPh HO H H OH H OH CH2OH 3PhNHNH2 CH2OH O HO H H OH H OH CH2OH D-fructose D-果糖 (8) Chain shortening: the Ruff degradation(降级) The Ruff degradation is used mainly for determination and synthesis of new sugars. (9) Chain lengthening: the Kiliani-Fischer synthesis This method is used for synthesis of new sugars. 4. Determination of the structure of monosaccharides (1) Fisher’s proof of the configuration of glucose D-glucose degradation degradation D-arabiose D-mannose HNO3 Optically active product COOH H OH H OH H OH COOH COOH HO H H OH H OH COOH D-erythrose HNO3 Meso-tartaric acid COOH H OH H OH COOH (2) Determination of Ring Size Approach 1 The size of the ring can be determined from the structure of the open-chain form Approach 2 An acetal of the monosaccharide is oxidized with excess HIO4 3 + CH3OH + HCOOH Problem: H H HIO4 HOHO O HO H H OCH3 OH H H3O+ 5. Disaccharides 二糖 Cellobiose 纤维二糖 Maltose 麦芽糖 Lactose 乳糖 1,1’- link Sucrose 蔗糖 Which is a nonreducing sugar? 6. Polysaccharides starch(淀粉) Amylose 直链淀粉 Amylopectin 支链淀粉 cellulose(纤维素) H H CH2OH O H H OH CH2OH O H H OH O C O H H OH H H OH The glycosidic linkages are b , 1: 4 n Blood type(血型) is determined by the nature of the sugar bound to the protein on the surface of red blood cells 7. Nuleic acids 核酸 核蛋白 nucleoprotein 核酸(RNA,DNA) Nucleic acid 蛋白质 protein 核苷酸(单体) Nucleotide 磷酸 Phosporic acid 核苷 Nucleoside 戊糖(2种:核糖, 脱氧核糖) pentose 碱基(5种) base 戊糖 pentose HOH2C H O H HO HOH2C OH H H O H HO H OH OH H H H 脱氧核糖 deoxyribose 核糖 ribose 核苷 Nucleoside base HO H O H H H OH OH 核糖核苷 ribonucleosides base HO H O H H H OH H 脱氧核糖核苷 deoxyribonucleosides 常见碱基(base) O OH O NH N N OH N H H2N H3C H2N N H N 鸟嘌呤 guanine (G) NH N N H OH O 胸腺嘧啶 thymine(T) NH2 NH2 N N N N H N N N O OH N N HN O 脲嘧啶 uracil (U) H3C OH OH N H 胞嘧啶 cytosine (C) NH NH2 N HN N N H N N N O 腺嘌呤 adenine (A) N H 在RNA中存在下列四种核苷: NH2 N CH2OH O OH 脲苷 N OH N NH O CH2OH O OH 胞苷 N OH O NH2 O O CH2OH O OH N OH 腺苷 N N N CH2OH O OH N NH N NH2 OH 鸟苷 规则:嘧啶的1位和嘌呤环的9位分别和核糖的苷羟基相连 核苷酸 ribonucleic acid 核苷的磷酸酯:核苷的3位或5位的羟基和磷酸结合 O O HO P OH N OCH2 O OH N H NH N O N NH2 N H HO H O H H H O OH O P OO- NH N NH2 O O O P O H O DNA deoxyribonuleic acids base H H H H O O P O H O base O H H H H O O P O H O base O H H H H O O P O H O base O H OH H H H Contents • • • • • • • • • • • • • • • • • • • Classification of carbohydrates Monosaccharides ★ ★ Classification ★ Structure chain: configuration: D,L-; erythro/threo; cyclic structure: α-,β-; mutarotation ★ Reactions oxidation: tollen’s reagent, Br2-H2O; HNO3; HIO4 reduction: NaBH4 formation of osazones formation of glycosides acylation etherification chain shorting (degradation) and lengthening Fischer’s proof of the configuration of glucose determination of ring size Disaccharides Polysaccharides Nucleic acids Assignments • 23-54, 57, 59, 63, 66, 67,69