Ch. 7 Ionic Bond Notes

IONIC COMPOUNDS AND METALS
A CHEMICAL BOND IS A FORCE THAT HOLDS
TWO ATOMS TOGETHER.
CAN FORM BETWEEN THE POSITIVE NUCLEUS
OF ONE ATOM AND THE NEGATIVE ELECTRONS
OF ANOTHER, OR BY THE ATTRACTION OF
POSITIVE AND NEGATIVE IONS.
WE WILL DISCUSS THE ATTRACTIONOF
POSITIVE AND NEGATIVE IONS IN THIS
CHAPTER.
The outermost electrons that are involved in chemical bonding.
Know these groups and their number of valence electrons:
Group 1 = 1
Group 2 = 2
Group 13 = 3
Group 14 = 4
Group 15 = 5
Group 16 = 6
Group 17 = 7
Group 18 = 8
NOTE: We will discuss the transition metals later
http://periodic.lanl.gov/images/periodictable.
Diagram used to keep track of valence electrons.
Examples:
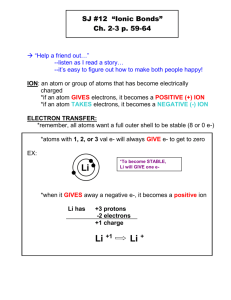
A positive ion forms when an atom loses one or more valence electrons in order to attain a noble gas configuration.
Noble gases are stable because they have 8 valence electrons (with the exception of helium)
A positive ion is known as a cation
In an ionic bond metals lose electrons to become stable. Therefore, groups 1, 2 , and
3-13 lose electrons during chemical bonding
Nonmetals easily gain electrons to attain a stable outer electron configuration.
An anion is a negatively charged ion that results from gaining one or more electrons.
Since metals lose electrons during ionic bonding and nonmetals gain electrons, we can assign charges to certain groups of the periodic table.
Group 1 = +1
Group 2 = +2
Group 13 = +3
Group 14 = skip
Group 15 = -3
Group 16 = -2
Group 17 = -1
Group 18 = no charge/stable configuration
An ionic bond forms when a metal atom donates electrons to a nonmetal atom.
The atom that donates electrons becomes positively charged, and the atom that received the electrons becomes negatively charged.
The electrostatic force that holds oppositely charged particles together in an ionic compound is referred to as an ionic bond.
(opposites attract)
In the chemical formula for any ionic compound the symbol of the cation is always written first followed by the symbol of the anion.
Subscripts, which are numbers to the lower right of a symbol, represent the number of ions of each element in an ionic compound.
If no subscript is written it is assumed to be one.
The sum of the oxidation numbers must equal zero because ionic compounds are neutral.
*NOTE: elements always combine in the lowest whole number ratio
K + and Cl -
Na + and O 2-
Mg 2+ and S 2-
Al 3+ and O 2-
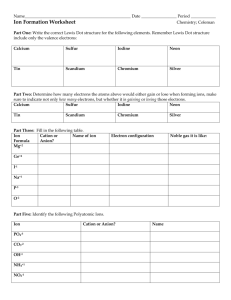
Ions made up of more than one atom. Refer to the common ion handout and the back of your periodic table for the polyatomic ion formulas.
Many ionic compounds contain polyatomic ions.
A polyatomic ion acts as an individual ion in a compound and its charge applies to the entire group of atoms. ***NEVER CHANGE THE
SUBSCRIPTS OF A POLYATOMIC ION!!
If more than one polyatomic ion is needed, place parentheses around the ion and write the appropriate subscript outside the parentheses.
Na + and NO
3
-
Ca 2+ and SO
4
2-
Fe 3+ and ClO
3
-
Cu 2+ and C
2
H
3
O
2
-
Ba 2+ and PO
4
3-
1.
2.
3.
4.
Name the cation followed by the anion.
Remember that the cation is always written first in the formula.
For monatomic (one atom) cations, use the element name.
For monatomic anions, use the root of the element name plus the suffix –ide.
Example: Na + and Cl -
When the compound contains a polyatomic ion, simply name the cation followed by the name of the polyatomic ion.
Example: Na + and C
2
H
3
O
2
-
5. To distinguish between multiple oxidation numbers of the same element, the name of the chemical formula must indicate the oxidation number of the cation. The oxidation number is written as a Roman numeral in parentheses after the name of the cation.
Examples:
Fe 2+ and O 2-
Fe 3+ and O 2-
Name the following compounds:
NaBr
CaCl
2
KOH
Cu(NO
3
)
2
Ag
2
CrO
4
In an ionic compound, large numbers of positive and negative ions exist together in a ratio determined by the number of electrons transferred from the metal atom to the nonmetal atom.
The ions pack into a regular repeating pattern to balance the attraction and repulsion between the ions. This forms a crystalline structure called a crystal lattice.
In the solid state, the ions in an ionic compound are locked into fixed positions by strong attractive forces. As a result, ionic solids do not conduct electricity.
However, when ions are allowed to move freely in a molten liquid state or when dissolved in water, ionic compounds will conduct electricity. This is called an electrolyte.
Because ionic bonds are relatively strong, ionic crystals require a large amount of energy to be broken apart.
As a result, ionic crystals have high melting and boiling points.
This is due to the strong attractive forces that hold the ions in place.
The energy required to separate 1 mol of the ions of an ionic compound is referred to as the lattice energy.
It is related to the size of the ions bonded and the charge of the ion.
Smaller ions produce greater attractions and higher lattice energies.
Example: the lattice energy of a lithium compound is greater than that of a potassium compound containing the same anion because the lithium ion is smaller than the potassium ion.
The charge of the ion affects the value of the lattice energy.
The greater the charge of the positive and negative ions, the greater the lattice energy.
Compound Lattice energy in kJ/mol
KI 632
KBr
RbF
671
774
NaI
NaBr
NaCl
682
732
769
Compound Lattice energy in kJ/mol
KF 808
AgCl
NaF
910
910
LiF
SeCl2
MgO
1030
2142
3795
Although metals are not ionic, they share several properties with ionic compounds.
Metals often form lattices
The electron sea model proposes that all the metal atoms in a metallic solid contribute their valence electrons to form a “sea” of electrons. This sea of electrons surrounds the metal cations in the lattice.
A metallic bond is the attraction of a metallic cation for delocalized electrons.
This gives metals their properties such as high melting points, malleability, ductility, durability, thermal and electrical conductivity, strength and hardness.