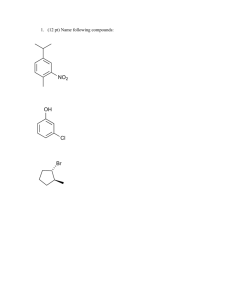
Ch. 15 - Philadelphia University
advertisement
. Reactions of Aromatic Compounds Based on Solomons , Fryhle Organic Chemistry 10th. Edition Created by Professor William Tam & Dr. Phillis Chang Ch. 15 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 15- 2 1. Electrophilic Aromatic Substitution Reactions Overall reaction H + E E + H + Ch. 15 - 3 X SO3H SO3 H2SO4 X2 FeX3 O O R R HNO3 Cl AlCl 3 H2SO4 RCl AlCl 3 NO2 R Ch. 15 - 4 2. A General Mechanism for Electrophilic Aromatic Substitution Different chemistry with alkene C C + Br2 + Br2 Br C C Br No Reaction Ch. 15 - 5 Benzene does not undergo electrophilic addition, but it undergoes electrophilic aromatic substitution H E A E +H A (H substituted by E) Ch. 15 - 6 Mechanism ● Step 1 + E E E slow r.d.s. E Ch. 15 - 7 Mechanism ● Step 2 E B H fast E + B H Ch. 15 - 8 Ch. 15 - 9 3. Halogenation of Benzene Benzene does not react with Br2 or Cl2 unless a Lewis acid is present (catalytic amount is usually enough) Ch. 15 - 10 Examples Cl Cl2 FeCl3 25o HCl + HBr (90%) Br Br2 FeCl3 heat + (75%) ● Reactivity: F2 > Cl2 > Br2 > I2 Ch. 15 - 11 Mechanism FeBr3 Br Br Br Br FeBr3 (weak electrophile) Br + FeBr4 (very reactive electrophile) Ch. 15 - 12 Mechanism (Cont’d) Br slow r.d.s. Br Br Br Ch. 15 - 13 Mechanism (Cont’d) Br H Br FeBr3 Br + Br H + FeBr3 Ch. 15 - 14 F2: too reactive, give mixture of mono-, di- and highly substituted products F2 Lewis acid F F F F + + F + others Ch. 15 - 15 I2: very unreactive even in the presence of Lewis acid, usually need to add an oxidizing agent (e.g. HNO3, 2+ Cu , H2O2) e.g. I2 I (86%) HNO3 I2 CuCl 2 I (65%) Ch. 15 - 16 4. Nitration of Benzene NO2 conc. HNO 3 conc. H2SO4 50-60oC + H3O+ (85%) Electrophile in this case is (nitronium ion) + HSO4 NO2 Ch. 15 - 17 Mechanism O HO S O O H + HO N O O H HSO4 + O O H O N O N O + H2O (NO2) Ch. 15 - 18 Mechanism (Cont’d) NO2 slow r.d.s. NO2 NO2 NO2 Ch. 15 - 19 Mechanism (Cont’d) NO2 H H2O NO2 + H3O+ Ch. 15 - 20 5. Sulfonation of Benzene Mechanism ● Step 1 SO3 + H3O+ + HSO4 2 H2SO4 ● Step 2 O O S slow O O O S O H other resonance structures Ch. 15 - 21 ● Step 3 O O S O fast O H S HSO4 O O + H2SO4 ● Step 4 O S O O fast S O H O H H O H O + H2O Ch. 15 - 22 Sulfonation & Desulfonation SO3, conc. H2SO4 o o 25 C - 80 C SO3H dil. H2SO4 H2O, 100oC Ch. 15 - 23 6. Friedel–Crafts Alkylation R X Lewis acid (e.g. AlCl3) R + HX R = alkyl group (not aryl or vinyl) Electrophile in this case is R o o ● R = 2 or 3 o ● Or R ClAlCl 3 (R = 1 ) Ch. 15 - 24 Mechanism Cl AlCl 3 Cl + AlCl 3 AlCl 4 Ch. 15 - 25 Mechanism (Cont’d) Ch. 15 - 26 Mechanism (Cont’d) H Cl AlCl 3 + HCl + AlCl3 Ch. 15 - 27 Note: Not necessary to start with alkyl halide, other possible functional groups can be used to generate a reactive carbocation e.g. + H+ via H+ Ch. 15 - 28 OH BF3 + via 60oC H O BF3 Ch. 15 - 29 7. Friedel–Crafts Acylation O Acyl group: R C O O AlCl3 + R Cl R 80oC Electrophile in this case is R–C≡O (acylium ion) Ch. 15 - 30 Mechanism O O + R Cl AlCl 3 R R C O C Cl AlCl 3 R C O Ch. 15 - 31 Mechanism (Cont’d) R O C O R O O R R Ch. 15 - 32 Mechanism (Cont’d) O R H O Cl AlCl 3 R + HCl + AlCl3 Ch. 15 - 33 Acid chlorides (or acyl chlorides) O R C Cl ● Can be prepared by SOCl2 O R C O OH or PCl5 R C Cl Ch. 15 - 34 8. Limitations of Friedel–Crafts Reactions When the carbocation formed from an alkyl halide, alkene, or alcohol can rearrange to one or more carbocations that are more stable, it usually does so, and the major products obtained from the reaction are usually those from the more stable carbocations Ch. 15 - 35 For example + (not formed) Cl AlCl 3 AlCl 3 (How is this Formed?) Ch. 15 - 36 Reason Cl + AlCl 3 1o cation (not stable) H AlCl 4 + 1,2-hydride shift H 3o cation (more stable) Ch. 15 - 37 Friedel–Crafts reactions usually give poor yields when powerful electron-withdrawing groups are present on the aromatic ring or when the ring bears an –NH2, –NHR, or –NR2 group. This applies to both alkylations and acylations CF3 These usually give poor yields in Friedel-Crafts reactions SO3H NH2 > R > OH O > N(CH3)3 O > > NO2 > Ch. 15 - 38 The amino groups, –NH2, –NHR, and –NR2, are changed into powerful electronwithdrawing groups by the Lewis acids used to catalyze Friedel-Crafts reactions H N H H H N AlCl 3 > + AlCl 3 Does not undergo a Friedel-Crafts reaction Ch. 15 - 39 Aryl and vinylic halides cannot be used as the halide component because they do not form carbocations readily Cl sp2 , AlCl 3 sp2 Cl , AlCl 3 No Friedel-Crafts reaction No Friedel-Crafts reaction Ch. 15 - 40 Polyalkylations often occur + OH BF3 + 60oC (24%) (14%) Ch. 15 - 41 9. Synthetic Applications of Friedel-Crafts Acylations: The Clemmensen Reduction Clemmensen ketone reduction O R Zn/Hg R HCl reflux Ch. 15 - 42 Clemmensen ketone reduction ● A very useful reaction for making alkyl benzene that cannot be made via Friedel-Crafts alkylations e.g. ? Ch. 15 - 43 Clemmensen ketone reduction ● Cannot use Friedel-Crafts alkylation Cl give AlCl 3 but NOT Ch. 15 - 44 Rearrangements of carbon chain do not occur in Friedel-Crafts acylations O O AlCl3 + R Cl R 80oC (no rearrangement of the R group) Ch. 15 - 45 O O Cl Zn/Hg conc. HCl reflux AlCl 3 Ch. 15 - 46 10. Substituents Can Affect Both the Reactivity of the Ring and the Orientation of the Incoming Group Two questions we would like to address here ● Reactivity ● Regiochemistry Ch. 15 - 47 ● Reactivity Y Y E faster or slower than E E E Y = EDG (electron-donating group) or EWG (electron-withdrawing group) Ch. 15 - 48 ● Regiochemistry Y Y Y Y E E E E (ortho) (o) (meta) (m) (para) (p) Statistical mixture of o-, m-, pproducts or any preference? Ch. 15 - 49 G G + E A Electrophilic reagent A substituted benzene E H other resonance structure Arenium ion Ch. 15 - 50 Z Y > > Z donates electrons The ring is more electron rich and reacts faster with an electrophile Y withdraws electrons The ring is electron poor and reacts more slowly with an electrophile Ch. 15 - 51 ● Reactivity Since electrophilic aromatic substitution is electrophilic in nature, and the r.d.s. is the attack of an electrophile (E ) with the benzene p-electrons, ⊖ an increase in e density in the benzene ring will increase the reactivity of the aromatic ring towards attack of an electrophile, and result in a faster reaction Ch. 15 - 52 ● Reactivity On the other hand, decrease in ⊖ e density in the benzene ring will decrease the reactivity of the aromatic ring towards the attack of an electrophile, and result in a slower reaction Ch. 15 - 53 ● Reactivity Increasing activity Y EDG –H EWG Ch. 15 - 54 ● Reactivity EDG (electron-donating group) on benzene ring Increases electron density in the benzene ring More reactive towards electrophilic aromatic substitution Ch. 15 - 55 ● Reactivity EWG (electron-withdrawing group) on benzene ring Decreases electron density in the benzene ring Less reactive towards electrophilic aromatic substitution Ch. 15 - 56 ● Reactivity towards electrophilic aromatic substitution EDG EWG > > Ch. 15 - 57 Regiochemistry: directing effect ● General aspects Either o-, p- directing or mdirecting Rate-determining-step is pelectrons on the benzene ring attacking an electrophile (E ) Ch. 15 - 58 Y E ortho attack Y Y E o-I Y E o-II E o-III Ch. 15 - 59 Y E meta attack Y m-I Y E m-II Y E m-III E Ch. 15 - 60 Y E para attack Y E Y p-I E Y p-II p-III E Ch. 15 - 61 If you look at these resonance structures closely, you will notice that for ortho- or parasubstitution, each has one resonance form with the positive charge attached to the carbon that directly attached to the substituent Y (o-I and p-II) Y E o-I Y p-II E Ch. 15 - 62 When Y = EWG, these resonance forms (o-I and p-II) are highly unstable and unfavorable to form, thus not favoring the formation of o- and pregioisomers, and m- product will form preferentially Ch. 15 - 63 On the other hand, if Y = EDG, these resonance forms (o-I and p-II) are extra-stable (due to positive mesomeric effect or positive inductive effect of Y) and favorable to form, thus favoring the formation of o- and p- regioisomers Ch. 15 - 64 Classification of different substituents Y Y (EDG) –NH2, –NR2 Strongly activating –OH, –O o-, p- directing –NHCOR –OR Moderately o-, pactivating directing –R (alkyl) –Ph Weakly activating o-, p- –H NA NA directing Ch. 15 - 65 Classification of different substituents Y Y (EWG) –Halide (F, Cl, Br, I) Weakly o-, pdeactivating directing –COOR, –COR, Moderately m–CHO, –COOH, deactivating directing –SO3H, –CN –CF3 , –CCl3 , –NO2 , –⊕NR3 Strongly mdeactivating directing Ch. 15 - 66 11. How Substituents Affect Electrophilic Aromatic Substitution: A Closer Look Ch. 15 - 67 11A. Reactivity: The Effect of Electron-Releasing and Electron-Withdrawing Groups If G is an electron-releasing group (relative to hydrogen), the reaction occurs faster than the corresponding reaction of benzene G G > > + E H G releases electrons. E Transition state is stabilized G > H E Arenium ion is stabilized When G is electron donating, the reaction is faster Ch. 15 - 68 If G is an electron-withdrawing group, the reaction is slower than that of benzene G + E H G withdraws electrons E Transition state is destabilized G > > G > H E Arenium ion is destabilized When G is electron withdrawing, the reaction is slower Ch. 15 - 69 Ch. 15 - 70 11B. Inductive and Resonance Effects: Theory of Orientation Two types of EDG OR NR2 (i) or CH3 > (ii) by positive mesomeric effect (donates electron towards the benzene ring through resonance effect) by positive inductive effect (donates electron towards the benzene ring through s bond) Ch. 15 - 71 Two types of EDG ● Positive mesomeric effect is usually stronger than positive inductive effect if the atoms directly attacked to the benzene ring is in the same row as carbon in the periodic table Ch. 15 - 72 Similar to EDG, EWG can withdraw electrons from the benzene ring by resonance effect (negative mesomeric effect) or by negative inductive effect O C CH3 e.g. Deactivate the ring by resonance effect > F > C> F F Deactivate the ring by negative inductive effect Ch. 15 - 73 11C. Meta-Directing Groups EWG EWG E (EWG ≠ halogen) E (major) EWG = –COOR, –COR, –CHO, –CF3, –NO2, etc. Ch. 15 - 74 For example CF3 NO2 (ortho) CF3 CF3 NO2 CF3 NO2 CF3 NO2 - H+ (ortho) (not favorable) NO2 (highly unstable due to negative inductive effect of –CF3) Ch. 15 - 75 CF3 (highly unstable due to negative inductive effect of –CF3) CF3 CF3 CF3 - H+ NO2 (para) NO2 NO2 NO2 CF3 (para) (not favorable) NO2 Ch. 15 - 76 (positive charge never attaches to the carbon directly attached to the EWG: –CF3) relatively more favorable CF3 NO2 (meta) CF3 CF3 NO2 CF3 NO2 NO2 CF3 - H+ (relatively more favorable than o-, p- products) (meta) NO2 Ch. 15 - 77 11D. Ortho–Para-Directing Groups EDG EDG EDG E E + E para ortho (major) EDG = –NR2, –OR, –OH, etc. Ch. 15 - 78 For example OCH3 NO2 (ortho) OCH3 OCH3 OCH3 NO2 NO2 OCH3 NO2 (ortho) (favorable) + -H OCH3 NO2 NO2 (extra resonance structure due to positive mesomeric effect of –OCH3) Ch. 15 - 79 OCH3 OCH3 OCH3 (para) - H+ NO2 NO2 NO2 NO2 OCH3 OCH3 OCH3 NO2 NO2 (extra resonance structure due to positive mesomeric effect of –OCH3) (para) (favorable) Ch. 15 - 80 OCH3 NO2 (meta) OCH3 (3 resonance structures only, no extra stabilization by positive mesomeric effect of –OCH3) less favorable OCH3 NO2 OCH3 NO2 NO2 OCH3 - H+ (meta) (less favorable) NO2 Ch. 15 - 81 For halogens, two opposing effects Cl > Cl negative inductive effect withdrawing electron density from the benzene ring positive mesomeric effect donating electron density to the benzene ring Ch. 15 - 82 Overall ● Halogens are weak deactivating groups Negative inductive effect > positive mesomeric effect in this case) Ch. 15 - 83 Regiochemistry Cl NO2 (ortho) Cl Cl Cl NO2 NO2 Cl NO2 (ortho) (favorable) + -H Cl NO2 NO2 (extra resonance structure due to positive mesomeric effect of –Cl) Ch. 15 - 84 Cl Cl Cl (para) - H+ NO2 NO2 NO2 NO2 Cl Cl Cl NO2 NO2 (extra resonance structure due to positive mesomeric effect of –Cl) (para) (favorable) Ch. 15 - 85 Cl NO2 (meta) Cl (3 resonance structures only, no extra stabilization by positive mesomeric effect of –Cl) less favorable Cl NO2 Cl NO2 NO2 Cl - H+ (meta) (less favorable) NO2 Ch. 15 - 86 11E. Ortho–Para Direction and Reactivity of Alkylbenzenes R R > > + E H R > E Transition state is stabilized H E Arenium ion is stabilized Ch. 15 - 87 Ortho attack CH3 Relatively stable contributor E CH3 CH3 E E > CH3 E Ch. 15 - 88 Meta attack CH3 E CH3 CH3 CH3 E E E Ch. 15 - 89 Para attack CH3 E E CH3 CH3 E E > CH3 Relatively stable contributor Ch. 15 - 90 12. Reactions of the Side Chain of Alkylbenzenes CH3 Methylbenzene (toluene) Ethylbenzene Isopropylbenzene (cumene) Phenylethene (styrene or vinylbenzene) Ch. 15 - 91 12A. Benzylic Radicals and Cations R H CH2 CH2 - RH Methylbenzene (toluene) C C The benzyl radical C Benzylic radicals are stabilized by resonance C Ch. 15 - 92 C LG C - LG A benzyl cation C C C Benzylic cations are stabilized by resonance C Ch. 15 - 93 12B. Halogenation of the Side Chain: Benzylic Radicals O O CH3 + N O NBS Br CCl4 light Br + Benzyl bromide (-bromotoluene) (64%) N H O N-Bromosuccinimide (NBS) furnishes a low concentration of Br2, and the reaction is analogous to that for allylic bromination Ch. 15 - 94 Mechanism ● Chain initiation X X peroxides heat or light 2X ● Chain propagation H C6H5 C H H H + X C6H5 + H C X H Ch. 15 - 95 ● Chain propagation H H C6H5 + C X X H C6H5 C X +X H ● Chain termination H H C6H5 + C H X C6H5 C X H Ch. 15 - 96 e.g. (more stable benzylic radicals) Br Br NBS + h (major) (very little) o (less stable 1 radicals) Ch. 15 - 97 13. Alkenylbenzenes 13A. Stability of Conjugated Alkenylbenzenes Alkenylbenzenes that have their side-chain double bond conjugated with the benzene ring are more stable than those that do not non-conjugated system C C conjugated system C is more stable than C C C Ch. 15 - 98 Example H+ OH heat (not observed) - Ha Ha Hb - Hb Ch. 15 - 99 13B. Additions to the Double Bond of Alkenylbenzenes HBr Br RO OR heat Br HBr (no peroxides) Ch. 15 - 100 Mechanism (top reaction) RO OR RO + H 2 RO + RO Br Br H Br + Br Br (more stable benzylic radical) Br + H Br (less stable) Br Ch. 15 - 101 Mechanism (bottom reaction) H H H Br (more stable benzylic cation) (less stable) Br Br Ch. 15 - 102 13C. Oxidation of the Side Chain O CH3 - 1. KMnO4, OH , OH 2. H3O+ (100%) Ch. 15 - 103 O 1. KMnO4, OH-, OH 2. H3O+ O 1. KMnO4, OH-, OH 2. H3O+ O 1. KMnO4, OH-, OH 2. H3O+ O O 1. KMnO4, OH-, 2. H3O+ OH Ch. 15 - 104 Using hot alkaline KMnO4, alkyl, alkenyl, alkynyl and acyl groups all oxidized to –COOH group o For alkyl benzene, 3 alkyl groups resist oxidation 1. KMnO4, OH-, 2. H3O+ No Reaction ● Need benzylic hydrogen for alkyl group oxidation Ch. 15 - 105 13D. Oxidation of the Benzene Ring R 1. O3, CH3CO2H 2. H2O2 O R OH Ch. 15 - 106 14. Synthetic Applications CH3 How? NO2 Ch. 15 - 107 CH3 group: ortho-, para-directing NO2 group: meta-directing CH3 CH3 CH3Cl conc. HNO 3 AlCl 3 conc. H2SO4 heat NO2 CH3 NO2 + Ch. 15 - 108 If the order is reversed the wrong regioisomer is given conc. HNO 3 CH3Cl conc. H2SO4 heat AlCl 3 NO2 CH3 NO2 NOT CH3 NO2 Ch. 15 - 109 COOH NO2 We do not know how to substitute a hydrogen on a benzene ring with a –COOH group. However, side chain oxidation of alkylbenzene could provide the –COOH group Both the –COOH group and the NO2 group are meta-directing Ch. 15 - 110 Route 1 CH3 conc. HNO 3 CH3Cl conc. H2SO4 heat AlCl 3 NO2 NO2 COOH 1. KMnO4, OH-, NO2 2. H3O+ Ch. 15 - 111 Route 2 CH3 COOH CH3Cl 1. KMnO4, OH-, AlCl 3 2. H3O+ COOH conc. HNO 3 NO2 conc. H2SO4 heat Ch. 15 - 112 Which synthetic route is better? ● Recall “Limitations of Friedel-Crafts Reactions, Section 15.8” Friedel–Crafts reactions usually give poor yields when powerful electronwithdrawing groups are present on the aromatic ring or when the ring bears an –NH2, –NHR, or –NR2 group. This applies to both alkylations and acylations Route 2 is a better route Ch. 15 - 113 Br Both Br and Et groups are ortho-, paradirecting How to make them meta to each other? Recall: an acyl group is meta-directing and can be reduced to an alkyl group by Clemmensen ketone reduction Ch. 15 - 114 O Cl AlCl 3 O Br Br2 FeBr3 Br Zn/Hg HCl, heat O Ch. 15 - 115 14A. Use of Protecting and Blocking Groups Protected amino groups ● Example NH2 NH2 ? Br Ch. 15 - 116 Problem Not a selective synthesis, o- and pproducts + dibromo and tribromo products NH2 NH2 Br2 NH2 + Br Br Br NH2 + + others Br Br Ch. 15 - 117 Solution Introduction of a deactivated group on –NH2 O NH2 CH3 H Cl pyridine N O CH3 (an amide) Ch. 15 - 118 The amide group is less activating than –NH2 group ● No problem for over bromination The steric bulkiness of this group also decreases the formation of o-product Ch. 15 - 119 NH2 NH2 Br O (hydrolysis of amide) Cl pyridine NHCOCH 3 Br2, FeBr3 1. H2SO4, H2O, 2. OH- NHCOCH 3 Br Ch. 15 - 120 NH2 NH2 Br Problem Difficult to get o-product without getting p-product Over nitration Ch. 15 - 121 Solution Use of a –SO3H blocking group at the p-position which can be removed later NH2 NH2 NO2 O 1. dil. H2SO4 100oC Cl 2. OH- pyridine NHCOCH 3 SO3 conc. H2SO4 HO3S 60oC NHCOCH 3 HNO3 H2SO4 HO3S NHCOCH 3 NO2 Ch. 15 - 122 14B. Orientation in Disubstituted Benzenes Directing effect of EDG usually outweighs that of EWG With two EDGs, the directing effect is usually controlled by the stronger EDG Ch. 15 - 123 Examples (only major product(s) shown) CH3 CH3 NO2 NO2 (i) CF3 CF3 OMe OMe COCH 3 Br OMe COCH 3 COCH 3 + (ii) Br Br Ch. 15 - 124 Cl Substitution does not occur to an appreciable extent between metasubstituents if another position is open Cl X NO2 O2N HNO3 Br Cl Cl + + H2SO4 Br Br Br NO2 62% 37% 1% Ch. 15 - 125 NHCOCH 3 NHCOCH 3 NO2 (iii) COOMe COOMe NO2 + NHCOCH 3 O2N COOMe Ch. 15 - 126 OCH3 CH3 (iv) Cl OCH3 OCH3 CH3 Cl CH3 + Cl Ch. 15 - 127 Cl Br (v) NO2 Cl Cl Br + NO2 NO2 Br Ch. 15 - 128 15. Allylic and Benzylic Halides in Nucleophilic Substitution Reactions H CH2X C C C 1o Allylic C C X C C H 1o Benzylic Ar C C C X 3o Allylic H X R' R 2o Allylic H Ar R R X R 2o Benzylic Ar C X R' 3o Benzylic Ch. 15 - 129 A Summary of Alkyl, Allylic, & Benzylic Halides in SN Reactions ● These halides give mainly SN2 reactions: H3C X R CH2 X R CH X R' ● These halides may give either SN1 or SN2 reactions: CH2 Ar CH2 X Ar CH X C C H X C C C R X R Ch. 15 - 130 A Summary of Alkyl, Allylic, & Benzylic Halides in SN Reactions ● These halides give mainly SN1 reactions: R R' C R" R R X Ar C R' X C C C X R' Ch. 15 - 131 16. Reduction of Aromatic Compounds H2/Ni slow benzene + cyclohexadienes H2/Ni fast cyclohexene H2/Ni fast cyclohexane Ch. 15 - 132 16A. The Birch Reduction Na NH3, EtOH benzene 1,4-cyclohexadiene Ch. 15 - 133 Mechanism Na etc. benzene benzene radical anion EtOH Na H H H cyclohexadienyl radical H H H etc. H H cyclohexadienyl anion etc. EtOH H H H H 1,4-cyclohexadiene Ch. 15 - 134 Synthesis of 2-cyclohexenones OCH3 OCH3 Li liq. NH 3 EtOH (84%) H3O+ H2O O 2-cyclohexenone Ch. 15 - 135 END OF CHAPTER 15 Ch. 15 - 136