nrosci-biosc 1070 & 2070 - Honors Human Physiology
advertisement

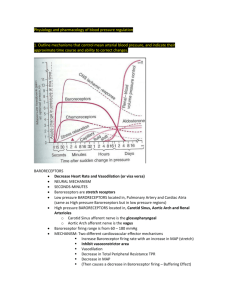
NROSCI-BIOSC 1070 MSNBIO 2070 September 28, 2015 Cardiovascular 5 Control of Blood Flow • Neural control of blood pressure • Local control of blood pressure • Hormonal control of blood pressure The Baroreceptor Reflex • Baroreceptors innervate the carotid sinus and aortic arch • Carotid sinus baroreceptors course in cranial nerve IX • Aortic arch baroreceptors course in a branch of cranial nerve X The Baroreceptor Reflex • When blood pressure increases, the baroreceptor terminals are stretched and the afferents fire more. • As with other types of stretch receptors, the responses of these afferents adapt to prolonged stretch. In the case of baroreceptors, this adaptation (resetting) begins within a few minutes. The Baroreceptor Reflex • Increases or decreases in blood pressure induce baroreceptor-mediated changes in sympathetic and parasympathetic activity • Baroreceptor activity displays “ceiling” and “floor” effects Brain Regions Mediating the Baroreceptor Reflex RVLM Rostral Ventrolateral Medulla Brain Regions Mediating the Baroreceptor Reflex CVLM Caudal Ventrolatera l Medulla Neural Basis of the Baroreceptor Reflex Some NTS neurons Baroreceptor afferents project directly to terminate in nucleus parasympathetic tractus solitarius preganglionic (NTS) in the neurons caudal in nucleus ambiguus medulla. (a region of the caudal medullary lateral reticular formation) Neural Basis of the Baroreceptor Reflex The RVLM is theare major NTS neurons also CVLM neurons region projectof tothe an area of the inhibitory, andbrainstem inhibit involved inventrolateral cardiothecaudal activity of neurons vascular control. The medullary reticular in the rostral ventroRVLM projects to formation (CVLM). lateral medulla (RVLM) sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the thoracic spinal cord. Baroreceptor Reflex Response of RVLM Neuron to Carotid Artery Stretch mm Hg 90 µV 100 200 100 0 -100 -200 Blood Pressure 80 Neural Activity 0 2 4 6 8 10 12 14 16 18 20 22 24 Time (sec) What Happens if the Baroreceptor Reflex is Dysfunctional? RVLM Neurons Integrate a Variety of Inputs The baroreceptor reflex provides a powerful mechanism to prevent sudden increases or decreases in blood pressure. However, additional mechanisms are needed to adjust blood pressure in the absence of perturbations. RVLM neurons integrate inputs from many regions of the nervous system, all of which contribute to altering blood pressure. Changes in Regional Perfusion During Exercise Blood flow to different organs changes appreciably during exercise Factors Adjusting Blood Flow to Different Vascular Beds Neural control (results in some patterning) Myogenic autoregulation (the ability of vascular smooth muscle to regulate its own activity) Response to paracrines from surrounding tissues (major factor producing increases in blood flow to metabolically active tissues) Hormonal control (epinephrine, vasopressin, angiotensin 2, aldosterone, atrial natriuretic factor) Myogenic Autoregulation Blood vessels automatically adjust their diameter in response to alterations in blood pressure, so that flow through the vascular bed remains constant. Ohm’s law (Q = ΔP/R) indicates that flow and perfusion pressure are directly proportional. As such, vascular resistance must increase in proportion to the increase in pressure to achieve autoregulation. Myogenic Autoregulation A rise in pressure stretches the wall of vascular smooth muscle cells, opening stretchsensitive Na+ channels, thereby resulting in a depolarization of the cell. This depolarization elicits an opening of voltage-gated Ca2+ channels on the surface, increasing Ca2+ and triggering vasoconstriction. Consequently, vessel diameter will become smaller as perfusion pressure rises. Autoregulation provides for a constant rate of O2 delivery regardless of perfusion pressure. Paracrine Control of Local Blood Flow Local control of vascular resistance is mainly controlled by the release of metabolites from tissues. H+ from acids (e.g. lactic acid), K+, and CO2 release promotes vasodilation. Low oxygen levels can also induce vasodilation, likely mediated by the release of adenosine from muscle cells during hypoxia. Each factor is additive, and the accumulation of all of these chemicals during exercise promotes the extensive increases in skeletal muscle perfusion that occur. Paracrine Control of Local Blood Flow Adenosine triggers an increase in cAMP, which activates protein kinase A (PKA). PKA phosphorylates and opens K-ATP channels, resulting in K+ efflux and hyperpolarization. Conductance through another K+ channel, the K-IR channel, increases when extracellular K+ rises, causing hyperpolarization of the cell. Increases in extracellular K+ due to metabolism of surrounding tissues also opens and increases K+ conductance through KIR channels. Hyperpolarization of smooth muscle causes a closing of voltage-gated Ca2+ channels, thereby resulting in relaxation of the smooth muscle. Through these mechanisms, there is profound vasodilation of vascular smooth muscle during exercise. Other Paracrine Factors that Regulate Blood Flow A large number of additional paracrine factors help to precisely regulate blood flow to particular vascular beds. One example is endothelin, which is released from damaged endothelial cells. Endothelin produces powerful vasoconstriction, which serves to reduce bleeding from damaged arteries. Release of serotonin from activated platelets induces vasoconstriction to prevent blood loss. In contrast, histamine release from healing tissues or mast cells promotes vasodilation. Endothelium-Derived Relaxing Factor Vasodilation is also produced by a chemical first called “endothelium-derived relaxing factor,” which is now know to be nitric oxide (NO). Sheer stress produced by the flowing of blood across the surface of endothelial cells opens mechanically-gated channels on the surface, including Ca2+ channels. Endothelium-Derived Relaxing Factor Ca2+ entering the endothelial cell through open channels combines with calmodulin (CM); the resulting Ca2+—CM complex activates NO synthase. An additional mechanotransduction mechanism initiates a kinase cascade, ultimately leading to phosphorylation of NO synthase and increased production of NO. Endothelium-Derived Relaxing Factor NO diffuses from endothelial cells to adjacent smooth muscle cells. NO produces smooth muscle relaxation by activating the enzyme guanylate cyclase, which results in increased levels of cyclic guanosine monophosphate (cGMP). Endothelium-Derived Relaxing Factor cGMP activates an ATPase that pumps calcium out of the smooth muscle cell, thereby inhibiting interactions between actin and myosin. Other factors besides sheer stress can also lead to NO production by endothelial cells: Bradykinin, an agent that is released during cellular damage. A number of products of metabolism Parasympathetic activity acting on vessels that receive this innervation (e.g., genitalia) Endothelium-Derived Relaxing Factor cGMP is rapidly broken down in vascular smooth muscle cells. In male genitalia, the degredation of cGMP is the result of the actions of phosphodiesterase type 5 (PDE5). cGMP can be degraded via other routes in other tissues. Drugs such as Viagra are phosphodiesterase type 5 inhibitors. Sexual stimulation in males results in parasympatheticallymediated vasodilation in the penis, via the local release of NO from endothelial cells in the corpus cavernosum. This NO release results in an increase in cGMP in smooth muscle cells of the corpus cavernosum, which generates vasodilation, an influx of blood, and an erection. Viagra prevents the cGMP from being rapidly degrated, so the vasodilation persists much longer than usual. Sheer Stress vs. Pressure Effects on Vessels Remember that sheer stress is tangential (parallel) to the vessel wall, while pressure is perpendicular to the wall. Sheer stress mainly affects the endothelial cells, while pressure affects the deeper layers of the vessel including the smooth muscle. Sheer stress and pressure can trigger different autoregulatory processes that oppose each other: o Acute increases in perfusion pressure can trigger smooth muscle cells to contract, reducing vessel diameter. o Reduced vessel diameter can raise the drag of blood along the vessel wall, leading to more sheer stress (and release of NO from endothelial cells). Thus, sheer stress can act as a negative modulator of myogeneic responses: sheer stress and blood pressure can induce opposing responses that keep each other in check. Sheer Stress vs. Pressure Effects on Vessels However, sheer stress responses can also have other roles. Vasodilation of muscle arterioles during exercise increases blood flow in the arterioles. The increased blood flow leads to sheer stress, and release of NO from endothelial cells in the arterioles. This leads to even more vasodilation. Hormonal Control of Blood Pressure • • • • Atrial Natriuretic Factor (Peptide) Vasopressin (Antidiuretic Hormone) Angiotensin II Aldosterone Atrial Natriuretic Factor (Peptide) • Atrial natriuretic factor is released from the atria when venous pressure (atrial stretch) is high. • This hormone tends to produce vasodilation. This will diminish cardiac return, and will thus decrease the workload of the heart which is overloaded with blood. • In addition, atrial natriuretic factor promotes secretion of water and salt by the kidney, to reduce blood volume. Atrial Natriuretic Factor (Peptide) Atrial Stretch Receptors • Both the atria and the pulmonary arteries contain stretch receptors, which are called low-pressure receptors. • These low-pressure receptors are much like arterial baroreceptors in structure, but because of their location do not sense pressure in the systemic circulation. Instead, they detect increases in pressure in the lowpressure parts of the circulation that are generated by increases in blood volume. Atrial Stretch Receptors • The axons of atrial stretch receptors project to the brainstem via the vagus nerve, and synapse in nucleus tractus solitarius (NTS). • Activation of atrial stretch receptors elicits a brainstem-mediated reflex that tends to an increase heart rate and probably contractility. Vasopressin • Signals from atrial stretch receptors are also transmitted to the hypothalamus, and affect the release of vasopressin. Signals from atrial stretch receptors can elicit a decrease in vasopressin release, which will in turn act on the kidney to result in lowered volume in the cardiovascular system. • Vasopressin is additionally released when the hypothalamus detects an increase in blood osmolarity, which occurs when blood volume decreases or the concentration of solutes increases. Vasopressin • A large decrease in blood pressure sensed by arterial baroreceptors triggers vasopressin release. • Increased blood levels of angiotensin II induce vasopressin release. Vasopressin • Increases in vasopressin result in kidney reabsorbing more water. As a consequence, blood volume increases and urine production decreases. • Drastic reductions in blood volume and blood pressure result in massive elevations in vasopressin levels, which induces vasoconstriction in some vascular beds. Vasopressin Posterior Pituitary Hormones • Posterior pituitary hormones are synthesized by neurons in the paraventricular and supraoptic nuclei of the hypothalamus • These hormones are released like neurotransmitters when the neurons fire • The release of the hormones is dependent on the number of neurons that fire and the rate and duration of their firing What Happens if Baroreceptors are Removed? • Following baroreceptor denervation, blood pressure is highly unstable. • However, mean blood pressure remains near 100 mmHg. • A mechanism other than the baroreceptor reflex must establish the blood pressure set-point. Renin-Angiotensin System •• Renin converts an inactive plasma protein made by the liver, Angiotensin II is a very potent vasoconstrictor substance. • angiotensinogen, When blood pressure is low, special into angiotensin I. cells in the kidney called juxtaglomerular cells (JG cells) detect the condition and • Angiotensin I is converted by an enzyme located on the release a peptide called renin.called angiotensin converting endothelium of blood vessels, • enzyme The sympathetic nervous system can also act toII.induce renin (ACE), into the active form, angiotensin This enzyme isrelease. concentrated in blood vessels of the lung. Other Functions of Angiotensin II • • It affects causes the the parts adrenal cortex to release the of the medulla that control potentiates the release of NE from sympathetic It stimulates thirst, the release and the of antidiuretic addition of water hormone to the hormone aldosterone, which causes the kidney to sympathetic outflow, to increase heart rate and terminals. (vasopressin) from the pituitary, which stimulates body reabsorb salt and water into the blood. The net vasoconstriction. water retention and an increase in plasma volume. result is that blood volume is increased. Summary of Angiotensin 2 Actions Summary of Angiotensin 2 Actions Not all Triggers of Ang Secretion are Equal! Clinical Note Widely-prescribed anti-hypertensive mechanisms include Angiotensin Converting Enzyme (ACE) inhibitors and Angiotensin II receptor antagonists Advantage of Angiotensin II Receptor Blockers Over ACE Inhibitors AT1 receptors mediate most of the effects of Ang-II on blood pressure. AT2 receptors are found in many tissues, including the uterus, ovary and several brain regions, but they are not known to be directly related to cardiovascular homeostasis. ACE-2 and Alternate Ang Pathways Red — Inhibitory Green — Excitatory Summary: Control of Blood Pressure Questions for Discussion What is a quadriplegic’s resting blood pressure, and how is it maintained? What happens when he/she is lifted out of bed? Question for Discussion How do anti-hypertensive medications work? 1. 2. 3. 4. 5. 6. 7. 8. Peripherally-acting alpha-receptor antagonist Centrally-acting alpha receptor agonist Beta-1 receptor antagonist Diuretic ACE inhibitor Angiotensin-2 receptor antagonist Calcium channel blocker Direct vasodilator MAP = (HR * (EDV-ESV)) * TPR