Osmosis and Diffusion Lab Handout
advertisement

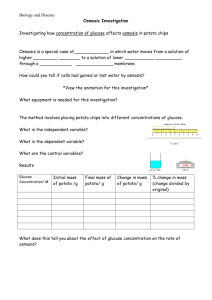
The Effect of Sugar Concentration on Percent Change in Mass in Potatoes Osmosis Lab The purpose of this lab is to test the effect of sugar concentration on the percent change in mass in potatoes. In this lab, you will be given six unknown solutions of sugar water - a 0.2 M, 0.4 M, 0.6 M, and a 0.8 M solution - and a potato. Your job is to do three things - to determine each solution’s molarity (concentration), to determine the molarity of a potato, and then explain how your procedure worked to give you your data. Diffusion is the movement of molecules from an area of high concentration to an area of low concentration. When water molecules diffuse across a semipermeable membrane - like a cell membrane - it is called osmosis. Cell membranes are semipermeable membranes in which molecules of a certain size can pass through and others cannot. Sucrose is a type of disaccharide that can be dissolved in water - it is too big to pass through a cell membrane. When one solution has a greater concentration of solute - like sucrose - than another solution, it is said to be hypertonic to the other. When one solution has a lesser concentration of solute than another solution, it is said to be hypotonic to the other. When two solutions have equal concentrations of solute, they are said to be isotonic to each other. Problem Solutions of 0.2 M, 0.4 M, 0.6 M, and 0.8 M concentrations of sugar water were made, but they were not properly labeled. There was also a container with just distilled water (0.0 M) mixed in without a label. Because the solutions are mixed, they were randomly assigned labels of A, B, C, and D. Question What are the concentrations of the unknown solutions? What is the concentration of the potato? Hypothesis _______________________________________________________________________________ _______________________________________________________________________________ Materials potato slices triple beam balance four solutions of unknown concentration Procedure 1. 2. 3. 4. Day 1 Label six cups to correspond with the solution labels: A, B, C, D, E, and F. Create six potato cores of nearly uniform size. Identify each core as A, B, C, D, E, and F. Using the triple beam balance, mass each core. Record the mass of each core. Place each potato core in its appropriate unknown solution and set aside until next class. Day 2 5. Next class, using a paper towel and without pressing down, roll the cores to remove excess water. 6. Mass each potato core again. Record the new mass of each potato core. 7. Calculate percent change in mass (See formula below). Record this value. 8. In a bar graph, graph each potato’s percent change in mass. 9. Using the percent change in mass, determine the molarity of each solution. 10. Draw a line of best fit through your data. Your potato’s molarity is equal to the point where your line of best fit crosses x-axis. Record this value. Data Table Potato Initial mass (g) Final Mass (g) % Change Mass A B C D E F Graph % Change in Mass = [(Final mass - Initial Mass) / Initial Mass] * 100 Solution Concentration (M) Discussion Questions 1. Why did some potatoes gain mass and some lose mass? Make sure to use vocabulary like hypertonic, hypotonic, or isotonic in your answer. 2. How did you determine the molarities of each solution from the % mass change? Make sure to use vocabulary like osmosis, high concentration, low concentration in your answer. 3. When you drew your line of best fit, why was the potato’s molarity at the point at which the line crossed the x-axis? 4. Why did you calculate the percent change in mass rather than using the change in mass from the potato cores? 5. A semi-permeable bag is filled with distilled water and then placed in a sucrose solution. The bag’s initial mass is 20 grams and its final mass is 17 grams. Calculate the percent change of mass, showing your calculations in the space below. Analyzing General Osmosis Lab Data Data Analysis Calculate the percent change in mass for the potatoes in each solution. % Change in Mass = [(Final mass - Initial Mass) / Initial Mass] * 100 Solution Initial Mass (g) Final Mass (g) A 22.8 18.4 B 14.3 16.6 Graphing Data Graph the calculated data in a bar graph. Label all axes and title your graph. % Change in Mass Diagram Draw arrows to indicate the direction of the water movement into or out of the potato. Analysis Questions 1. If osmosis is the movement of water from high concentration to low concentration, label the areas of high concentration and low concentration in both beakers above. 2. Was Solution A hypotonic, hypertonic or isotonic to the potato? Explain. 3. Was Solution B hypotonic, hypertonic or isotonic to the potato? Explain.