RedOx - OurTeachersPage.com
advertisement

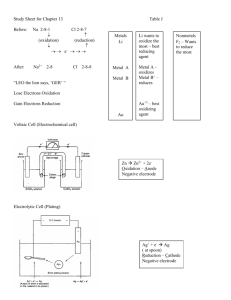
Loss of electrons Oxidation Gain of electrons Reduction 1. 2. 3. 4. Free elements = 0 Simple ions = charge F always -1 O nearly always -2, except when bonded to F, or in a peroxide 5. H nearly always +1, except when bonded to a metal. 6. Sum of the oxidation #’s in a neutral cmpd = 0 7. Sum of the oxidation #’s = charge for a polyatomic ion 8. For a covalent cmpd, the more electronegative element is assigned the negative oxidation # and vice versa Rules for Assigning Oxidation Numbers Involve the transfer of electrons Redox Reactions Occur simultaneously. # of electrons lost = # of electrons gained. Oxidation & Reduction LOSS of ELECTRONS = OXIDATION. GAIN OF ELECTRONS = REDUCTION LEO goes GER Single Replacement Synthesis Decomposition Redox Reactions Single Replacement: element + compound new element + new compound Synthesis: 1 product Decomposition: 1 reactant Recall Formats Have to assign oxidation numbers to everything in the equation. The ones that change are redox. Identifying Redox Reactions Shows either the oxidation or reduction reaction, including the electrons gained or lost. Half-Reaction 1) Conservation of matter 2) Conservation of charge Half-Reactions must obey Total charge on LHS of equation = Total charge on RHS of equation What does conservation of charge mean? Electron term is on the product side. Oxidation Half-Reation Oxidation Half-Reaction H2 + 2H + 2e Electron term is on the reactant side. Reduction Half-Reation Reduction Half-Reaction 2+ Zn + 2e Zn 1) S S2- + 2e2) Cl2 Cl- + e3) Mn7+ + 3e- Mn4+ 4) Ca2+ Ca + 2eWhich half-reaction shows conservation of mass & conservation of charge? H2, N2, O2, F2, Cl2, Br2, I2 Pesky diatomics H2, N2, O2, F2, Cl2, Br2, I2 You have to keep the subscript in the half-reaction! What’s the problem with the Pesky Diatomics? H2, N2, O2, F2, Cl2, Br2, I2 You have to keep the subscript in the half-reaction! + 2H 2e H2 + O2 + 4e- 2O2- a) b) c) d) Fe2+ Fe + 2eFe + 2e- Fe2+ Fe Fe2+ + 2e2+ Fe + 2e Fe Which half-reaction is correct for reduction? a) b) c) d) Ca2+ + 2e- Ca Ca2+ Ca + 2eCa + 2e- Ca2+ 2+ Ca Ca + 2e Which half-reaction is correct for oxidation? a) b) c) d) O2 O2 O2 O2 + 2e- O2 2O2- + 2e+ 4e- 2O22 2O + 4e Which half-reaction is correct for the reduction of O2? Helps something else get oxidized by itself being reduced. Oxidizing Agent Helps something else get reduced by itself being oxidized. Reducing Agent 4 3 2 1 0 -1 -2 -3 -4 2) And if you’re lucky you strike oil & it shoots up 1) You dig down with an oil rig 1. Assign all oxidation #’s. 2. Use oil rig to figure out what’s oxidized & what’s reduced. 3. Write the half-reactions. 4. Add half-reactions, multiplying to adjust electrons if necessary. 5. Transfer coefficients & balance remaining elements. Steps in Balancing Redox Eqs. Use Table J! If the stand-alone element is above the similar element in Table J, the reaction will occur. How do you predict if a given redox reaction will occur? Compare Li with Al – both are metals. Li > Al so reaction occurs. Li + AlCl3 ? Compare I2 with Cl – both are nonmetals. I2 < Cl2 so reaction DOES NOT occur. I2 + NaCl ? Galvanic or Voltaic (NYS–electrochemical) Spontaneous rxn Electrical energy Electrolytic Electrical energy Nonspontaneous rxn 2 kinds of cells in electrochemistry? Uses a spontaneous reaction to produce a flow of electrons (electricity). Exothermic. Galvanic/Voltaic/Electrochemic al (NYS) Cell Redox reaction is arranged so the electrons are forced to flow through a wire. When the electrons travel through a wire, we can make them do work, like light a bulb or ring a buzzer. So the oxidation & reduction reactions have to be separated physically. Galvanic Cell Use a spontaneous single replacement redox reaction to produce a flow of electrons. Electrons flow from oxidized substance to reduced substance. Galvanic/Voltaic/Electrochemical - 2 half-cells, each with a container, an aqueous solution, & an electrode connected by a - Wire and a - Salt Bridge Parts of a Galvanic Cell Surface at which oxidation or reduction half-reaction occurs. Electrode The anode is the metal that’s higher in table J. It’s more easily oxidized. Anode/Cathode in Galvanic Cell Anode to Cathode. Direction of electron flow (wire) Galvanic Cell – remember opposites attract. Direction of electron flow (wire) Anode to Cathode. Direction of positive ion flow (salt bridge) Electrode where oxidation occurs Anode Electrode where reduction occurs Cathode AN Ox ate a RED CAT Works for ALL cells Memory Aid Allows for migration of ions between half-cells. Necessary to maintain electrical neutrality. Reaction will not proceed without salt bridge. Salt Bridge Electrode where electrons originate. (higher in table J) Negative Electrode / Galvanic Cell Electrode that attracts electrons. (lower in table J) Positive Electrode / Galvanic Cell Electron flow wire Al = anode - Al+3 Positive ion flow Salt bridge Pb+2 & NO3-1 & NO3 Draw galvanic cell with Al & Pb -1 Pb = cathode Oxidation: Al Al3+ + 3eMetal electrode – Loses mass Aluminum ions in solution – concentration Reduction: Pb2+ + 2e- Pb Lead ions in solution – Concentration Metal electrode – gains mass Write the half-reactions for the previous cell Overall Rxn 2(Al Al+3 + 3e-) 3(Pb+2 + 2e- Pb) _____________________________ 2Al + 3Pb+2 2Al+3 + 3Pb Concentration of Zn2+ ions. Generally [ ] means concentration of whatever is inside [ ]. What does [Zn2+] mean? Galvanic Cells provide a voltage. They are a type of battery. Uses of Galvanic Cells 1. Plate metals on other metals 2. Prepare column 1 and column 17 elements from compounds 3. Recharge batteries Uses of ELECTROLYTIC CELLS Uses a flow of electrons (electricity) to force a nonspontaneous reaction to occur. Endothermic. Electrolytic Cell 1. Its got a power supply – a battery! 2. You don’t have two separate containers. How do you identify an electrolytic cell in a picture or diagram? 1) Fused salt cell 2) Plating Cell What are the 2 kinds of electrolytic cells you are responsible for? An Ox ate a Red Cat. Anode is still oxidation. Cathode is still reduction How do you label the anode & cathode in an electrolytic cell? Use the battery. The electrode attached to the positive pole of the battery is positive & vice versa. How do you label the positive & negative electrodes in an electrolytic cell? A POX on electrolytic cells! Anode Oxidation Positive What’s the memory trick for remembering the polarity of electrolytic cells? Putting a very thin layer of one metal on top of another metal! What is electroplating? + Battery + - Loses mass 3) Element to be plated. Cu+2 and SO4-2 1) See battery so it’s electrolytic! 2) Trace the + & - signs back to electrodes. 4) Anode = Oxidation Cu Cu+2 + 2e5) Cathode = Object to be Plated = Reduction Cu+2 + 2e- Cu Gains mass Notice: Net reaction is just moving Cu around. Electrolytic cell used to prepare group 1 & group 17 elements from their compounds (salts). What is a fused salt cell? MOLTEN What does fused mean? + - Anode Cathode Na+ Anode = Oxidation. 2Cl- Cl2 + 2e- Cathode = Reduction. Na+ + e- Na ClMolten NaCl Fused Salt Cell: Opposites Attract!