Part 2 of Osmosis/Diffusion lab
advertisement

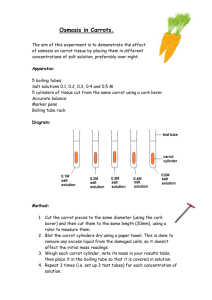
AP Biology Cerveny 2015-2016 Diffusion Lab: Part 2 Osmosis Through a Membrane Learning Objectives: 1. To design an experiment to measure water potential in plant cells. 2. To analyze the data collected in experiments and make predictions about molecular movement through cellular membranes. 3. To work collaboratively to design experiments and analyze results. 4. To connect the concepts of diffusion and osmosis to the cell structure and function. All life takes place in water – either external water or internal water – so we need to address the special case of water movement across cell membranes while studying diffusion. The diffusion of water across a semi-permeable membrane is called osmosis. As with the diffusion of solutes, water moves from a region of higher concentration to a region of lower water concentration. The free energy associated with the water in this system is called water potential (). Distilled water has the highest concentration of water and at standard conditions has = 0 bars (a bar is a measurement of pressure). The concentration of water decreases as solutes – like sugars and salts – dissolve in the water. Accordingly, the water potential of the solution decreases. Using the principles you learned in the first part of this lab, we will investigate the movement of water in and out using a model cell, followed by testing in real plant cells. From the data you acquire you will generate a curve related to concentration of a sucrose solution then use that curve to determine the water potential in a sample of plant tissue (aka baby carrots). Key Vocabulary: Define these words in your lab notebook: Water Potential Solute Potential Turgor Pressure Key Equations: w = solute + pressure solute = -iCRT i = ionization constant C = molar concentration of solute R = pressure constant, 0.0831 (L*bars)/(moles*K) T = temperature (273 + oC) Percent Change = ((original – final)/original) x 100 For this lab your numbers will be the appropriate masses. Pre-lab questions: 1. What is kinetic energy, and how does it differ from potential energy? 2. What environmental factors affect kinetic energy and diffusion? 3. Why are gradients important in diffusion and osmosis? 4. What would happen if you applied saltwater to a freshwater plant? 5. How does a plant cell control its internal (turgor) pressure? AP Biology Cerveny 2015-2016 Materials: Graduated cylinder Ruler Carrot samples Plastic wrap plastic cups dialysis tubing scissors Mystery solutions balances Distilled water Safety: The materials in this laboratory are safe. Don’t run with the scissors or poke your eye out with the carrot. When finished the solutions can be poured down the drain and the carrots discarded in the collection bucket (I’ll put them in my yard waste) Determining sucrose concentrations in mystery solutions: 1. Day 1 a. Cut a 6 pieces of dialysis tubing from the roll. Each piece should be ~15 cm long. b. Soak the tubing pieces in water until they are soft enough to work with. Tie one end of each bag off like you are tying a balloon. c. To each piece of tubing add enough of a single mystery solution to fill it a little more than halfway. Repeat with the other 5 solutions. d. Tie off the end of the tubing (leave some space for expansion) and blot it dry with paper towels. e. Mass the samples. Record in a table in your notebook. f. Place the samples into different plastic cups that are arranged on the white paper towels. Write each sample’s weight on the appropriate cup. g. Add 0.5 M sucrose until the samples are clearly floating in the cup. h. Cover the cups with plastic wrap and let sit overnight. 2. Day 2 a. Carefully remove samples from the beakers and blot dry. Mass each sample and record it. This is your final mass. b. Determine the percent change in mass from your original mass. Plot this data using the sucrose concentrations of: 0, 0.2, 0.4, 0.6, 0.8, and 1.0 M. You will need to think about which percent change would go with which concentration. Show ALL of your calculations AP Biology Cerveny 2015-2016 Determining the sucrose concentration of a carrot sample: 1. Day 1 a. Pour ~50 mL of each mystery solution into 6 separate plastic cups. Make them deep enough that the carrot is fully covered. b. Obtain 6 baby carrots, blot them dry and determine their mass. c. Place one carrot into each of the mystery solution containers. Keep track of which carrot is in which solution so you can measure the changes in mass. d. Cover the cups with plastic wrap and let sit overnight. 2. Day 2 a. Make any observations you would like to of the carrots in the cups. b. Carefully remove the carrots, blot them dry and determine their mass. c. Determine the % change in mass of the carrots from their original. Plot this data against the sucrose concentrations from above and determine the concentration of sucrose in the carrot. Show ALL your calculations. Analysis: answer these questions in your lab notebook 1. What is the solute potential of the 0.5 M sucrose solution. 2. If you looked at the carrot cells under the microscope, describe what you think you would see.