AP Biology Lab 2
advertisement

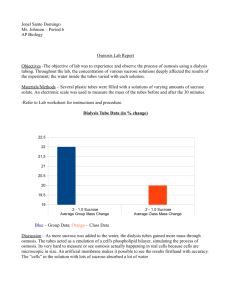
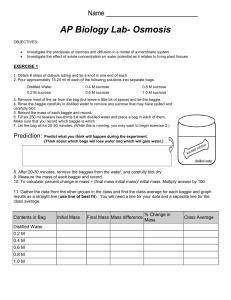
Osmosis Lab Purpose: To investigate the effect of solute concentration on osmosis as it relates to living plant tissues. Background: Research what osmosis is and various osmotic solutions. Make sure to cite your resource(s) in MLA or APA format. Hypothesis: Write in if, then form. Materials: Various fruits/vegetables, knife, 5 cups, 5 sucrose solutions, food coloring, labeling tape, digital balance, calculators, distilled water Procedure: 1. Use slicing device to cut 6 French fry size pieces of vegetable/fruit. 2. Obtain 5 sucrose solutions in different cups and label. 3. Take initial mass of vegetable/fruit pieces. 4. Place vegetable/fruit pieces in cup for 24 hours. 5. Blot each of the 6 pieces off. Take final mass of pieces. Calculate mass difference and % change in mass. To calculate: percent change in mass = (final mass-initial mass)/ initial mass. Multiply answer by 100. Contents in Bag Initial Mass Final Mass Mass difference % Change in Mass Distilled Water 0.2 M 0.4 M 0.6 M 0.8 M 1.0 M ANALYSIS OF RESULTS Graph data, identify variables and constants! Then answer the following questions in paragraph form: 1. Explain the relationship between the change in mass and the molarity of sucrose. 2. Why did you calculate the percent change in mass rather than simply using the change in mass? 3. Describe which solutions were hypotonic, hypertonic, and isotonic. Be sure to explain why.