FP_OsmosisLab.doc
advertisement

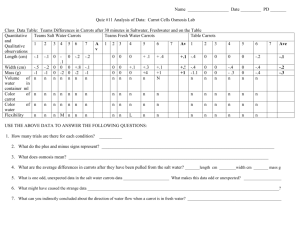
Osmosis Lab Biologist: _____________________________ Period: ___________ Date:__________ Materials: baby carrots, 1.0 M sucrose solution, DI water, scales, beaker or cups, calculator Part 1 Background 1. Describe diffusion. 2. Does net diffusion stop? Explain. 3. Does diffusion stop? Explain. 4. Define molarity? How is it calculated? 5. Define hypotonic, isotonic, and hypertonic. Procedures 1. Collect 3 baby carrots. Your instructor will tell you which solutions you are testing in the lab today. ___________________________ 2. Mass all 3 baby carrots. Be sure each carrot is dried. Record the initial mass in your data table. 3. Prepare your solutions. a. The unknown solution is prepared for you. You need to add ________ mL of the unknown solution. b. To prepare your standard solution you will need to calculate the dilution from a 1.0 M solution of sucrose. Look at the information from your equation sheet. You need to prepare __________ mL of standard solution. Show your calculation below. Volume of 1.0 M sucrose solution needed: _________________ Volume of distilled water needed: ____________________ 4. Show your instructor your calculation. 5. Prepare your solution. Be sure to swirl and mix your solution. 6. Gently place carrots in the solutions. Be sure you know which carrots are going into which solution! 7. Store your carrots in solution overnight. 8. Remove your carrots from the solution. 9. Dry the carrots just before you mass them. 10. Record the final mass in your data table. 11. Calculate the mass difference. Mass difference = Final mass- Initial mass. Record the value in your data table. 12. Calculate the percent change in mass. Percent change in mass = (Final mass - Initial mass) x 100 Initial mass 13. Repeat steps 8-12 with your second solution. 14. Share your data with your instructor and classmates. Group Data Table Solution Initial Mass Final Mass Mass Difference Percent Change in Mass Percent Change in Mass Carrot #1 Percent Change in Mass Carrot #1 Percent Change in Mass Carrot #1 Mean Class Data Table Solution 0.1 M 0.3 M 0.6 M 0.9 M Graph 1. Determine the independent variable from the experiment. The independent variable is the variable that we control in the experiment. This will be the x-axis. 2. Determine the dependent variable from the experiment. The dependent variable is the outcome variable we are testing for in the experiment. This will be the y-axis. 3. To create the graph determine the range of your data and determine a scale before doing anything else. You will be graphing the class data. 4. Plot the independent variable and dependent variable. Identify the unknown solutions with colored points. You will need to create a legend to identify which color goes with each unknown solution. Show your instructor. 5. Make a best fit line using the data. Analysis Questions 1. Use your graph to estimate the molarity of the unknown solutions. Explain how you estimated the molarity. 2. Describe the trend of the percent change in mass as the molarity of the solution increased. 3. Estimate the molarity of the solution where the percent change in mass is equal to zero. What is happening at this point? Conclusion Questions 1. When should you use a bar graph (like the histogram from the variation lab)? Explain. 2. When should you use a line graph (like the in this lab)? Explain. 3. Explain how or why some carrots lost mass and some carrots gained mass. 4. Why did you calculate and graph the percent change in mass rather than the change in mass? Explain. 5. Cell membranes are nonpolar. From chemistry we know polar molecules such as water cannot mix with or cross a nonpolar barrier such as the cell membrane. How is it possible for water to move into or out of a cell? 6. Identify three sources of error that could have occurred in this lab. Explain how you could correct for the error or minimize the error in future experiments.