Non-invasive papillary urothelial carcinoma
advertisement

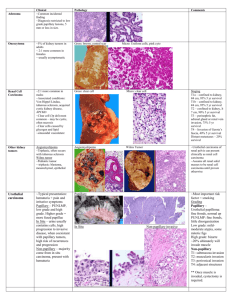
Bladder Cancers Mitra Heidarpour, MD Associate Professor of Pathology Isfahan University of Medical Sciences Epidemiology • Male female ratio slightly less than 3:1 • 2/3 over the age of 65, rare under age 30 • Overwhelmingly a sporadic disease Risk Factors • • • • • • • • • • Genetic and environmental factors Occupational exposure Aniline dyes Cyclophosphamide Tobacco exposure Ionizing Radiation Chronic Infection [stone, catheter] Phenacetin ingestion dietary fat Schistosomia hematobium Bladder Cancer - Pathology • Over 90% of lesions are transitional cell carcinoma • 5-7% of lesions are pure squamous cell carcinoma – Associated with chronic irritation [stones, catheter, Schistosomiasis] • 1-2% Adenocarcinoma – Rule out metastatic source. • Metaplastic elements of squamous or adenocarcinoma in TCC are different than pure tumor devoid of TCC Morphologic features: Urothelial Carcinoma • Multicentrity is common. – They are consequence of intramucosal seeding of a single tumor rather than true multicenteric tumors. Bladder cancer: Entities • 75-85% superficial bladder cancer pTa, pTis, pT1 • 10-15% muscle-invasive bladder cancer pT2, pT3, pT4 • 5% metastatic bladder cancer N+, M+ Classification of Papillary Urothelial Neoplasms No absolute agreement WHO 1973 Papilloma Grade 1 Grade 2 Grade 3 WHO/ISUP 98-99 Papilloma PULMP and low grade low and high grade high grade WHO (2004)/ISUP Classification of Urothelial Tumors • Papillary Urothelial Lesions – – – – – Papillary hyperplasia Urothelial papilloma Papillary urothelial neoplasm of low malignant potential Low-grade papillary urothelial carcinoma High-grade papillary urothelial carcinoma • Flat Urothelial Lesions – – – – – Flat uothelial hyperplasia Reactive (inflammatory) atypia Urothelial atypia of unknown significance Dysplasia Carcinoma in situ WHO (2004)/ISUP Classification of Urothelial Tumors • Non-invasive urothelial neoplasias – Urothelial papilloma – Urothelial papilloma, inverted type – Non-invasive papillary urothelial neoplasm of LMP – Non-invasive papillary urothelial carcinoma (low grade) – Non-invasive papillary urothelial carcinoma (high grade) WHO (2004)/ISUP Classification of Urothelial Tumors • Infiltrating urothelial carcinoma • with squamous differentiation with glandular differentiation with squamous and glandular differentiation Nested variant Microcystic variant Micropapillary variant Lymphoepithelioma-like carcinoma Lymphoma-like variant Plasmacytoid variant Sarcomatoid Giant cell variant Undifferentiated carcinoma Squamous neoplasms • Squamous cell carcinoma • Verrucous squamous cell carcinoma Glandular neoplasms • Adenocarcinoma • Urachal carcinoma • Clear cell adenocarcinoma • In situ adenocarcinoma Neuroendocrine tumors • Small cell carcinoma • Paraganglioma • Carcinoid Mesenchymal and miscellaneous tumors Rhabdomyosarcoma Leiomyosarcoma Angiosarcoma Osteosarcoma Malignant fibrous histiocytoma Leiomyoma Neurofibroma Haemangioma Malignant melanoma Lymphoma Urothelial Papilloma • • • • • • • • Benign condition Rare typically occurring as a small, isolated growth commonly in younger patients A discrete papillary growth with a central fibrovascular core lined by urothelium of normal thickness and normal cytology simple branching pattern without fusion The umbrella cell layer is often prominent and may show prominent vacuolization, nuclear enlargement, or cytoplasmic eosinophilia Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP) • markedly thickened (hyperplastic) urothelial lining • minimal to absent cytologic atypia • normal polarity of the urothelial cells with an orderly, predominantly linear arrangement perpendicular to the basement membrane. • Infrequent mitotic figures , usually limited to the basal layer. • Maintained umbrella cell layer • simple branching pattern of papilla • increased risk of developing recurrent or new papillary lesions which occasionally are of higher grade and may progress. • The diagnosis of PUNLMP should be carefully reconsidered in the presence of stromal invasion because that finding would be highly unusual. Papillary Urothelial Carcinoma, Low-Grade • Overall orderly appearance but with easily recognizable variation of architectural and or cytologic features seen at scanning magnification. • architecture is frequently complex with obvious anastomosis of adjacent papillae creating fused, confluent formations • Variation of polarity and nuclear size, shape, and chromatin texture • Mitotic figures are infrequent and usually seen in the lower half; but may be seen at any level of the urothelium Papillary Urothelial Carcinoma, High-Grade • Complex, disordered architecture • A spectrum of pleomorphism ranging from moderate to marked • The individual neoplastic cells are often more rounded than in lower grade lesions • Loss of polarity in relation to the basement membrane • Frequent mitotic figures, including atypical forms • Much higher risk of progression than low-grade lesions • High risk of association with invasive disease at the time of diagnosis. • A spectrum of cytologic and architectural abnormalities may exist within a single lesion, stressing the importance of examining the entire lesion and noting the highest grade of abnormality. WHO/ISUP Classification of Flat Intraurothelial Lesions • Hyperplasia • Flat lesions with atypia – Reactive (inflammatory type) – Atypia of unknown significance – Dysplasia (low-grade intraurothelial lesion) – Carcinoma in situ (high-grade intraurothelial lesion) Carcinoma In Situ (High-grade Intraurothelial Neoplasia) • characterized by the presence of cells with large, irregular, hyperchromatic nuclei that may be either present in the entire thickness of the epithelium or only a part of it. • an umbrella cell layer may still be present • The urothelium may be denuded (discohesive cells) • it may be diminished in thickness or hyperplastic • frequent mitoses • the lamina propria is frequently hypervascular and inflamed Immunoprofiles for CIS, Reactive Atypia, and Normal Urothelium CK 20 p53 CD44 CIS Cytoplasmic staining in full thickness (81%) Strong nuclear reactivity in full thickness (57%) Non-reactive; loss of normal basal staining Reactive atypia Reactive only in umbrella cells Non-reactive Diffuse membranous staining in full thickness Normal urothelium Reactive only in umbrella cells Non-reactive Membranous staining in basal cell layer only Invasion to muscle is of great consequence because of its influence on therapy 1. Care should be exercised not to misinterpret the inconsistent fascicles of muscularis mucosa (particularly in women) as belong to muscularis properia. 2. Care should be exercised not to misinterpret the mature adipose tissue that present in lamina propria as perivesical fat 3. Some times lymphocytic infiltration obscure the epithelial component of urothelial carcinoma (specially squamous ones) Lymphadenectomy • Improved survival with number of nodes • sampled 10-14 nodes suggested as cut point. Immunohistochemical feature 1. Consistent expression of CK20 (in contrast with morphologically similar TCC of ovary). Coordinate expression of ck7 & ck20 is particularly reproducible feature of UC (particularly in metastatic foci) 2. Trombomodeline is very sensitive but positive in most SCC & mesotheliomas 3. Uroplakin III is very specific but moderately sensitive 4. CEA, cathepsin B, CA19-9-CD15, CD10,… & TTF1 5. P53 in high proportion of UC (particularly high grade ones) and correlates well with prognosis. High grade UC vs Prostatic carcinoma UC+: Ck34 β E 12, ck7, P53 Prostatic carcinoma+: PSA, PSAP, leu7 UC versus inverted papilloma –ki67, p53 & ck20 (all of them positive in urothelial carcinoma) Staging of bladder cancer • • • • • • • • • • • T0: No evidence of a primary tumor Ta: Non-invasive papillary carcinoma Tis: Non-invasive flat carcinoma (or CIS) T1: The tumor has grown into the connective tissue . It has not grown into the muscle layer of the bladder. T2a: The tumor has grown into the inner half of the muscle layer. T2b: The tumor has grown into the outer half of the muscle layer. T3: The tumor has grown into the fatty tissue. T3a: microscopically. T3b: to be seen on imaging tests or felt by the surgeon. T4a: The tumor has spread to the stroma of the prostate (in men), or to the uterus and/or vagina (in women). T4b: The tumor has spread to the pelvic wall or the abdominal wall. • Bladder cancer can sometimes affect many areas of the bladder at the same time. If more than one tumor is found, the letter m is added to the appropriate T category. Nodes • lymph nodes near the bladder (in the true pelvis) and those along the blood vessel called the common iliac artery. These lymph nodes are called regional lymph nodes. Any other lymph nodes are considered distant lymph nodes. • NX: Regional lymph nodes cannot be assessed due to lack of information. • N0: There is no regional lymph node spread. • N1: The cancer has spread to a single lymph node in the true pelvis. • N2: The cancer has spread to 2 or more lymph nodes in the true pelvis. • N3: The cancer has spread to lymph nodes that lie along the common iliac artery. Metastasis • M0: There are no signs of distant spread. • M1: The cancer has spread to distant parts of the body. The most common sites are distant lymph nodes, the bones, the lungs, and the liver). Stages of bladder cancer • • • • • • Stage 0a (Ta, N0, M0) Stage 0is (Tis, N0, M0) Stage I (T1, N0, M0) Stage II (T2a or T2b, N0, M0) Stage III (T3a, T3b, or T4a, N0, M0) Stage IV (T4b, N0, M0 or Any T, N1 to N3, M0 or Any T, any N, M1) The pathology report of a bladder biopsy should include the following informations: 1. Grade 2. Configuration (papillary or solid) 3. Depth of penetration 4. Presence of muscle 5. Lymphatic invasion 6. Blood vessel invasion 7. Margins and TNM staging in cystectomy specimens