Section 12.1
advertisement

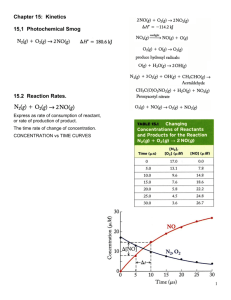
CHAPTER 12: KINETICS Chem 1212 Dr. Aimée Tomlinson Section 12.1 Reaction Rates Reaction Rate The rates at which products are formed and reactants are consumed are connected They are always represented as concentration change / time change For the general case aA + bB cC + dD the relationships are: Rate 1 [ A] 1 [ B] 1 [C ] 1 [ D] a t b t c t d t where t = tf - ti, [A] is the concentration of A (moles/L) and [A] = [A]f - [A]i we loose reactants as products are formed which is why the rates of A & B are negative Example Application of Definition 1. What is the rate relationship between the production of O2 and O3? 2O3(g) 3O2(g) [O3 ] 1 [O3 ] 1 [O2 ] 3 [O3 ] [O2 ] 2 [O2 ] or 2 t 3 t 2 t t t 3 t the rate of O2 production is 1.5 times faster than the ate of consumption of O3 the rate of O3 consumption is 2/3 times the production of O2 2. The decomposition of N2O5 proceeds according to the equation: 2N2O5(g) 4NO2(g) + O2(g) If the rate of decomposition of N2O5 at a particular instant is 4.2 x 10-7 M/s, what is the rate of production of NO2 and O2? 1 [ N 2O5 ] 1 [ NO2 ] [O2 ] 2 t 4 t t [ NO2 ] 4 [ N 2O5 ] 2 4.2 107 M 8.4 107 M s s t 2 t [O2 ] 1 [ N 2O5 ] 2.1107 M s t 2 t Rate Example: 2 N 2 O5( g ) 4 NO2( g ) O2( g ) rate of decomposition of N 2 O5 rate of formation of O 2 [ N 2O5 ] 0.0101 0.0120 M 1.9 10 5 M s t 400 300 s [O2 ] 0.00049 0.0040 M 9 106 M s t 400 300 s rate of formation of NO 2 [ NO2 ] 0.0197 0.0160 M 3.7 105 M s t 400 300 s Different Types of Rates Average Rate Concentration change over a time interval (colored region in plot) Instantaneous Rate Slope of the tangent line at a given time (purple line in plot) Section 12.2 Rate Law & Reaction Order Rate Law Relates the rate directly to reactant concentrations For the General case aA + bB cC + dD, the rate law is: Rate = k[A]m[B]n where m and n are determined experimentally k is called the rate constant CAUTION!!! Rate law is NOT related to stoichiometry! 2 N 2 O5( g ) 4 NO2( g ) O2( g ) CHCl3( g ) Cl2( g ) CCl4( g ) HCl( g ) Rate k[ N 2 O5 ] Rate k [CHCl3 ][Cl2 ] 1 2 Reaction Order The power to which each reactant is raised For the General case aA + bB cC + dD, with Rate = k[A]m[B]n “m” is the order of reactant A and “n” is the order of reactant B Overall reaction order is the sum of all or m+n in this case Name the reactant orders and overall reaction order for Rate k[CHCl3 ][Cl2 ] 1 2 It is 1st order in CHCl3 and ½ order for Cl2 with 1 ½ order overall Section 12.3 Experimental Determination of Rate Law Illustrative Example Determine the rate law, the rate constant and the reaction orders for each reactant and the overall reaction order using the data given below. Experiment [A]0 [B]0 Initial Rate M/s 1 2 3 0.100 0.100 0.200 0.100 0.200 0.100 4.0 x 10-5 4.0 x 10-5 16.0 x 10-5 Rate constant k & Overall Order It is an indicator of the overall rate order of the equation rate k A n M s ? M M ? M n s M 1 ? n sM s M n 1 order m k units zeroth 0 M /s first 1 1/ s second 2 1/ M s Final Thoughts What to do when finding ‘m’ isn’t obvious m 1 1 1 1 ln m ln 4 2 4 2 1 ln 4 m 2 1 ln 2 Section 12.4 & 12.5 First-Order Integrated Rate Law & its Half-Life First-Order Integrated Rate Law [ A] rate k[ A] t [ A] k t [ A] [ A ]t t d [ A] kdt [ A] o [ A ]0 ln[ A] |[[ AA]]0t kdt |t0 ln[ A]t ln[ A]0 kt ln[ A]t kt ln[ A]0 • Final equation is the integrated rate law • Change in reactant concentration over time may be plotted to get rate law • We plot ln[A]t versus t: ln[ A]t k t ln[ A]0 y m x b Half-Life for First-Order Reaction [ A]t ln kt [ A]0 1 2 [ A]0 ln kt 1 2 [ A]0 1 ln kt 1 2 2 0.693 kt 1 t 1 0.693 2 k 2 Section 12.6 Second-Order Reactions Second-Order Integrated Rate Law rate k[ A]2 [ A] t [ A] k t 2 [ A] [ A ]t t d [ A] kdt 2 [ A ]0 [ A] o 1 [ A]t |[ A]0 kdt |t0 A 1 1 kt At A0 1 1 kt At A0 • Final equation is the integrated rate law • This time we plot 1/[A]t versus t to get linearity 1 kt 1 [ A]t m x [ A] y b Section 12.7 Zeroth-Order Reactions Zeroth-Order Integrated Rate Law [ A] rate k [ A] k t t [ A ]t [ A ]0 t d [ A] kdt [ A] |[[ AA]]0t kdt |t0 [ A]t [ A]0 kt o [ A]t kt [ A]0 Summary of Integrated Equations Section 12.8 Reaction Mechanisms Definitions Reaction Mechanisms: how electrons move during a reaction Intermediate Species that is produced then consumed Never partakes or appears in the rate law Elementary Step A step that occurs in a reaction Most reactions have multiple elementary steps The stoichiometry of these steps CAN be used to get the reaction order The sum of these steps leads to the overall reaction Molecularity Number of reacting particles in an elementary step Uni- (1 species), Bi- (2 species), Ter- (3 species) Example Application Given the mechanism below state the overall reaction, rate law and molecularity of each step as well as the intermediate. step1: NO2 NO2 NO NO3 elementary step 2 : NO3 CO NO2 CO2 NO2 CO NO CO2 Rate law for step 1: rate1 = k1[NO2]2 Rate law for step 2: rate2 = k2[NO3][CO] Both steps have 2 interacting species so bimolecular Intermediate is NO3 which is produced then consumed elementary overall Section 12.9 Rate Laws & Reaction Mechanisms Examples of Molecularity Molecularity Elementary step Rate Law Unimolecular A products Rate = k[A] Bimolecular A + A products Rate = k[A]2 Bimolecular A + B products Rate = [A][B] Termolecular A + A + A products Rate = k[A]3 Termolecular A + A + B products Rate = k[A]2[B] Termolecular A + B + C products Rate = k[A][B][C] Example Problem What is the molecularity & rate law for each of the following? molecularity Rate law H 2 NO N H 2 O bimolecular rate k[ H 2 ][ NO ] N NO N 2 O bimolecular rate k[ N ][ NO] H 2 O H 2O bimolecular rate k[ H 2 ][O] Section 12.10 Rate Laws for Overall Reactions Rate Determining Step The slowest step in a mechanism Just like the slowest person in a relay race – the slowest step in the mechanism will ruin the speed/time of the reaction If the slow step is first it is very easy to get the rate law: k1 step 1: 2 NO2 NO NO3 ( slow) k2 step 2 : NO3 NO O2 ( fast ) the rate law would then be: rate k1 [ NO2 ]2 Example for Fast Step First What is the rate law for the mechanism below? k1 step1: NO O2 NO3 ( fast ) k2 step 2 : NO NO3 2 NO2 ( slow) Fast Equilibrium Applied Example The rate laws for the thermal and photochemical decomposition of NO2 are different. Which of the following mechanisms are possible for thermal and photochemical rates given the information below? Thermal rate = k[NO2]2 Photochemical rate = k[NO2] slow a.) NO2 NO O fast O NO2 NO O2 fast b.) NO2 NO2 N 2 O4 slow N 2 O4 NO NO3 fast NO3 NO O2 slow c.) NO2 NO2 NO NO3 fast NO3 NO O2 Section 12.11 Reaction Rates & Temperatures – Arrhenius Equation Arrhenius Equation Relates rate to energy and temperature k Ae Ea RT Frequency Factor A Energy of Activation, Ea Indicative of the number of successful collisions Energy that must be overcome to form products Section 12.12 Using the Arrhenius Equation Finding the Ea through plotting Mechanisms & Energy Profiles slow NO2 NO2 NO NO3 fast NO3 NO O2 14_19.jpg The slow step has the larger Ea since it takes longer to generate more energy Transition State Defn: state at which reactant bonds are broken and product bonds begin to form Located at the top of the hump for each elementary step in the reaction profile Section 12.13 & 12.14 Catalysts Catalyst A species that lowers the activation energy of a chemical reaction and does not undergo any permanent chemical change it is not present in the overall reaction expression it is not present in the rate law it must be consumed and produced in the elementary steps Two types of catalysts: Homogeneous: in the same phase as the reactants Heterogeneous: a different phase from reactants Example of Catalysis Uncatalyzed mechanism blue line in the figure hv step 1: O3 O2 O step 2 : O O3 2O2 2O3 3O2 Ea 17.7 kJ / mol Cl Catalyzed mechanism red line 14_21.jpg step 1: Cl O3 O2 ClO step 2 : ClO O3 Cl 2O2 2O3 3O2 Ea 2.2 kJ / mol