Problems du Jour
advertisement

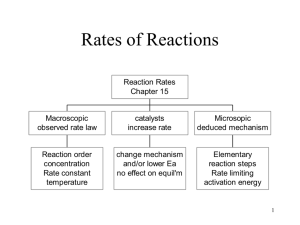
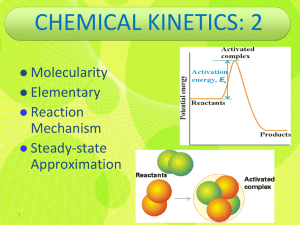
Reaction Mechanisms Consider a case where reactants have sufficient energy to cross the activation barrier to form products..... How does the reaction occur? How many bonds are formed/broken? How many steps are involved? This is the reaction mechanism Consider the reaction NO(g) + O3(g) NO2(g) + O2(g) (important in ozone destruction!) This reaction is thought to occur as the result of a single collision between an NO and an O3 molecule What are the necessary conditions for this to occur? 72 Similarly, the gas phase rearrangement reaction CH3NC(g) CH3CN(g) Occurs as the result of a single collision between H3CNC and another molecule Processes which occur in a single step (i.e., one collision) are called elementary steps (or elementary processes) Note: elementary processes are very rare!! Most reactions take place in a number of steps! Definition: Mechanism = series of elementary steps which add to give the observed stoichiometric reaction Steps in a mechanism are always elementary processes! 73 E.g., The mechanism for the stoichiometric reaction NO2(g) + CO(g) NO(g) + CO2(g) Appears to involve two elementary steps: (1) NO2(g) + NO2(g) NO3(g) + NO(g) (2) NO3(g) + CO(g) NO2(g) + CO2(g) Add these steps together: what happens? NO3 is an intermediate in this reaction- it is formed in one step, then consumed in a subsequent step 74 Molecularity of elementary steps Molecularity = number of reactant molecules participating in an elementary step 1 molecule: unimolecular E.g., CH3CN CH3NC 2 molecules: bimolecular E.g., NO2(g) + NO2(g) NO3(g) + NO(g), or NO3(g) + CO(g) NO2(g) + CO2(g) 3 molecules: termolecular (rare! why?) 75 E.g., consider the following mechanism for the conversion of ozone into O2: (1) O3(g) O2(g) + O(g) (2) O3(g) + O(g) 2O2(g) Write the overall equation for the reaction Are there any intermediates? What is the molecularity of each step in the mechanism? 76 Rate laws of elementary steps Rate laws for stoichiometric chemical reactions MUST be determined experimentally (E.g. method of initial rates) However, the rate law of any elementary step is based directly on its molecularity, e.g., Unimolecular: A products First order: Rate = k[A] Bimolecular: A + B products Second order: Rate=k[A][B] A + A products Second order: Rate = k[A]2 Termolecular: rate law for A+B+C prod ? 77 Remember: reaction order for each reactant in an elementary step = number of reactant molecules in that step E.g., write the rate laws for the elementary steps in the ozone - O2 example above Rate laws of multistep mechanisms What if one elementary step in a multistep mechanism is slower than the other(s)? E.g. suppose that one step has a larger Ea (and hence a smaller k) 78 The slow step is called the rate-determining step The overall reaction rate cannot exceed the rate of the slowest (rate-determining) step Therefore: the rate law for the rate-determining step determines the rate law for the entire mechanism! Examples: The following mechanism has been proposed for the gasphase reaction of H2 with ICl: H2(g) + ICl(g) HI(g) + HCl(g) k1 k2 HI(g) + HCl(g) I2(g) + HCl(g) Write the balanced equation for the overall reaction. Identify any intermediates in the mechanism. If the first step is slow and the second step fast, what rate law is predicted by this mechanism? 79 Note that, when k2>>k1, intermediate is consumed as fast as it is produced.... What problem arises if the second (or subsequent) step is slow? A multistep mechanism will always involve intermediates If the second step is slow, the rate law will contain an intermediate Why is this not a good thing? E.g., the stoichiometric reaction 2NO(g) + Cl2(g) 2NOCl(g) Was found experimentally to obey the rate law Rate = k[NO]2[Cl2] The following mechanism has been proposed: NO(g) + Cl2(g) k1 NOCl2(g) + NO(g) NOCl2(g) k2 80 2NOCl(g) (a) What rate law would be predicted by this mechanism if the first step were rate-limiting? Does this rate law agree with experiment? Note the appearance of intermediate in the rate law: is there a way of dealing with this? Assume equilibrium in the first step, i.e., it is reversible 81 Problems du Jour The following mechanism has been proposed for the gasphase reaction between CHCl3 and chlorine: (1) Cl2(g) 2Cl(g) fast (2) Cl(g) + CHCl3(g) HCl(g) + CCl3(g) slow (3) Cl(g) + CCl3(g) CCl4(g) fast (a) What is the overall reaction? (b) What are the intermediates in the mechanism? (c) What is the molecularity of each of the steps? (d) What is the rate law predicted by this mechanism? 82