Answer key 1 - Anthony Rohm
advertisement
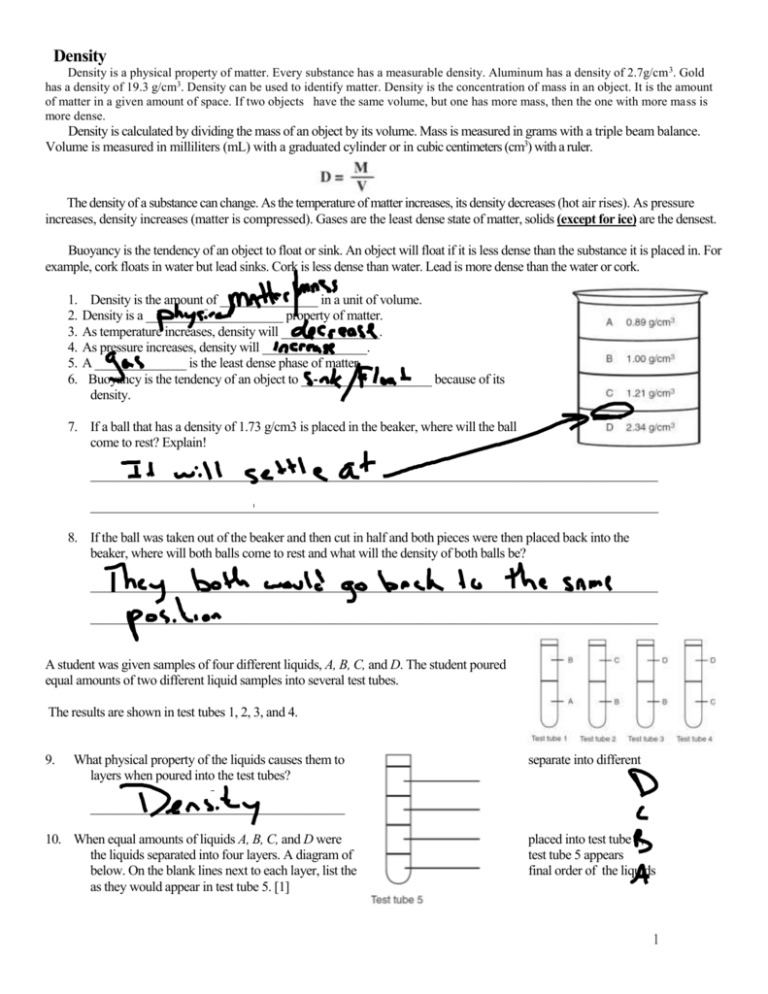
Density Density is a physical property of matter. Every substance has a measurable density. Aluminum has a density of 2.7g/cm 3. Gold has a density of 19.3 g/cm3. Density can be used to identify matter. Density is the concentration of mass in an object. It is the amount of matter in a given amount of space. If two objects have the same volume, but one has more mass, then the one with more mass is more dense. Density is calculated by dividing the mass of an object by its volume. Mass is measured in grams with a triple beam balance. Volume is measured in milliliters (mL) with a graduated cylinder or in cubic centimeters (cm3) with a ruler. The density of a substance can change. As the temperature of matter increases, its density decreases (hot air rises). As pressure increases, density increases (matter is compressed). Gases are the least dense state of matter, solids (except for ice) are the densest. Buoyancy is the tendency of an object to float or sink. An object will float if it is less dense than the substance it is placed in. For example, cork floats in water but lead sinks. Cork is less dense than water. Lead is more dense than the water or cork. 1. 2. 3. 4. 5. 6. Density is the amount of _______________ in a unit of volume. Density is a _____________________ property of matter. As temperature increases, density will _______________. As pressure increases, density will ________________. A ______________ is the least dense phase of matter. Buoyancy is the tendency of an object to ____________________ because of its density. 7. If a ball that has a density of 1.73 g/cm3 is placed in the beaker, where will the ball come to rest? Explain! _______________________________________________________________________________________ _______________________________________________________________________________________ 8. If the ball was taken out of the beaker and then cut in half and both pieces were then placed back into the beaker, where will both balls come to rest and what will the density of both balls be? _______________________________________________________________________________________ _______________________________________________________________________________________ A student was given samples of four different liquids, A, B, C, and D. The student poured equal amounts of two different liquid samples into several test tubes. The results are shown in test tubes 1, 2, 3, and 4. 9. What physical property of the liquids causes them to layers when poured into the test tubes? separate into different _______________________________________ 10. When equal amounts of liquids A, B, C, and D were the liquids separated into four layers. A diagram of below. On the blank lines next to each layer, list the as they would appear in test tube 5. [1] placed into test tube 5, test tube 5 appears final order of the liquids 1 11. Below is a chart with the density of 4 liquids. Using the letter of the liquid and its density, place the liquids in the correct order in the beaker on the blank line. LIQUID DENSITY Letter Density (g/mL) A 2.3 B C D 4.0 1.6 3.4 4 12. Take a look at the two boxes. Each box has the same volume. If each molecule (ball) has the same mass, which box would be more dense? Why? _____________________________________________________________ _____________________________________________________________ 13. How would cutting both blocks in half affect the density of each block? _______________________________________________________________________________________________ 14. If this object were placed in the beaker from question 11 where would it settle? Mass = 45.0 g Volume = 15.0 cm3 _______________________________________________________________________________________ a. If the object in question 14 is compacted to half its size it will probably sink to the bottom. Explain why this would happen. ______________________________________________________________________________________________ _____________________________________________________________________________________________ 15. Based on table A what is the density of the aluminum block? (Show all work on 16. In which of the liquids in Table B would an aluminum block float? _______________________________________________________________________________________ _______________________________________________________________________________________ 17. How would the density of a 50.0 cm3 aluminum block compare with that of the block described in Table A above. _______________________________________________________________________________________ _______________________________________________________________________________________ 2 18. Based on the diagram to the right, why is ICE an exception to the rule when it comes to density? _____________________________________________________________________________ _____________________________________________________________________________ 19. Which liquid in the diagram is the least dense? (1) wood (2) water (3) iron (4) mercury 20. Which solid in the diagram is the most dense? (1) wood (2) water (3) iron (4) mercury 21. What effect does an increase in temperature have on the density of air? Explain your answer. __________________________________________________________________________________________ __________________________________________________________________________________________ 22. The mineral sample shown is Galena. The chart lists the densities of several different minerals. Mineral Sample Galena Mass = 210 grams Mineral Gypsum Orthoclase Quartz Calcite Dolomite Fluorite Density (g/cm3) 2.3 2.6 2.7 2.7 2.9 3.2 Mineral Hornblende Chalcopyrite Pyrite Magnetite Galena Copper Density (g/cm3) 3.2 4.2 5.0 5.2 ? 8.9 a. What instrument can be used to measure the mass Galena? ________________________________________________________________ b. 50 mL of water is added to a 100 mL graduated cylinder. The Galena is added to the graduated cylinder and the volume increases to 78ml. What is the volume of the piece of Galena? c. Find the density of Galena if the mass is 210 grams. Show your work below and explain your choice and round to the nearest tenth. d. A different mineral has a mass of 40.0 grams and a volume of 8.0 ml. This sample is most likely the mineral ________________ Show your work below and explain your choice 3 Use the information below to answer questions 24-27. A student measures the masses and volumes of four pieces of metal. The results are shown below. Property Metal Metal Metal A B C Metal D Mass 10.0 g 10.0g 30.0 g 40.0 g Volume 2.0 cm3 5.0 cm3 5.0 cm3 8.0 cm3 Density ____________ ___________ ___________ __________ ____ __ _ _ 23. Calculate the density for each sample in the table above. The answer must be rounded to the nearest 0.1 g/cm3 ________ and the units must also be included. 24. Which metal is the densest? _______________________________________________________________________________________ 25. Which two pieces of metal might be made of the same metal? Explain. _______________________________________________________________________________________ _______________________________________________________________________________________ 26. Draw the density of each object (water is 1.0 g/cm3) in the fish tank. Hint is Block A (Block a.) 1.0 g/cm3 (Block b) 0.5 g/cm3 (Block c) 0.8 g/cm3 (Block d) 0.2 g/cm3 (Block e) 1.2 g/cm3 A 27. Estimate the densities of each substance below if each of the substances were cut in half. a)__________________________b)_________________________c)___________________________ d)__________________________e)_________________________ 4






