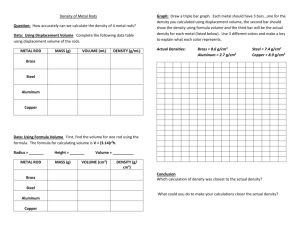
Density Worksheet 1
advertisement

Name: Period: Density Problems Directions: Show ALL your work…equation used, set-up with units and answer with correct units and significant digits. (Use the “GUESS” Method!!!) 1. What is the density of an object that has a mass of 126.7g and a volume of 70.0 cm3? 2. What is the density of an object that measures 1.0mm height, 42.01 cm length and 13.22 cm width? The mass is 145.2g. (Be careful, you must convert the height in mm to cm before doing the rest of the problem) 3. What is the volume of germanium whose mass is 14.0g and a density of 5.323g/cm3? 4. What is the mass of a copper sample whose volume is 14.0cm3 and a density of 8.95g/cm3? 5. A 30.0 sample of silver has a density of 10.49 g/cm3. What is its volume? 6. A piece of metal with a mass of 147 g is placed in a 50 mL graduated cylinder. The water level rises from 20mL to 41mL. What is the density of the metal? 7. What is the volume of a sample that has a mass of 0.020 kg and a density of 4 g/mL? (Make sure that you convert the kg to g) 8. A student finds a shiny piece of metal that she thinks is aluminum. In lab, she determines that the metal has a volume of 245 cm3 and a mass of 612 g. Is the metal aluminum? (Aluminum’s density is 2.70 g/cm3) 9. A 532 mL sample of water has a mass of 5 g. What is the density of water in g/L? (You must convert the mL to L) 10. You are converting 68 km to meters. Your answer is 0.068 m. Explain why this answer is incorrect and the likely source of the error. 1. Did you show all of your work? _______ (Yes or No) 2. Did you follow the “GUESS” Method? _______ (Yes or No) 3. Did you look back in your notes to see how to do the metric conversions for those problems which needed conversions? _______ (Yes or No)