WS 3
advertisement
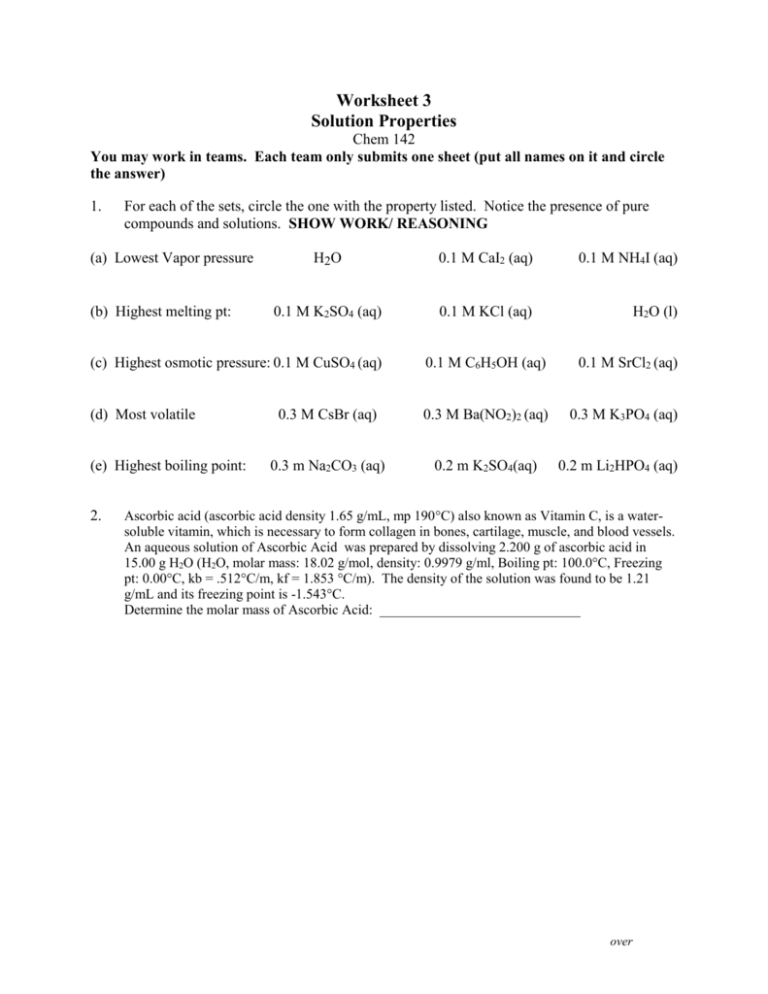
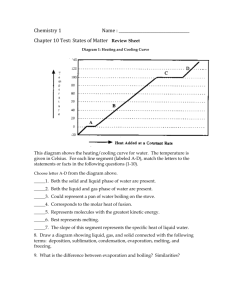
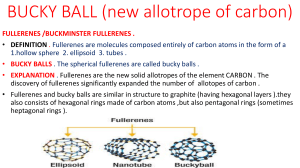
Worksheet 3 Solution Properties Chem 142 You may work in teams. Each team only submits one sheet (put all names on it and circle the answer) 1. For each of the sets, circle the one with the property listed. Notice the presence of pure compounds and solutions. SHOW WORK/ REASONING (a) Lowest Vapor pressure (b) Highest melting pt: H2O 0.1 M CaI2 (aq) 0.1 M NH4I (aq) 0.1 M K2SO4 (aq) 0.1 M KCl (aq) H2O (l) (c) Highest osmotic pressure: 0.1 M CuSO4 (aq) 0.1 M C6H5OH (aq) 0.1 M SrCl2 (aq) (d) Most volatile 0.3 M CsBr (aq) 0.3 M Ba(NO2)2 (aq) 0.3 M K3PO4 (aq) 0.3 m Na2CO3 (aq) 0.2 m K2SO4(aq) (e) Highest boiling point: 2. 0.2 m Li2HPO4 (aq) Ascorbic acid (ascorbic acid density 1.65 g/mL, mp 190C) also known as Vitamin C, is a watersoluble vitamin, which is necessary to form collagen in bones, cartilage, muscle, and blood vessels. An aqueous solution of Ascorbic Acid was prepared by dissolving 2.200 g of ascorbic acid in 15.00 g H2O (H2O, molar mass: 18.02 g/mol, density: 0.9979 g/ml, Boiling pt: 100.0°C, Freezing pt: 0.00°C, kb = .512°C/m, kf = 1.853 °C/m). The density of the solution was found to be 1.21 g/mL and its freezing point is -1.543°C. Determine the molar mass of Ascorbic Acid: _____________________________ over Chem 142 2. Fullerenes represent the newest allotrope of carbon. The discovery and isolation of fullerenes in 1985 ultimately resulted in a Noble Prize in Chemistry awarded to Kroto and Smalley. The most well known fullerene is Buckminster Fullerene, (Bucky Ball), which has the shape of a soccer ball. Bucky Ball has the chemical formula C60 (C60, molar mass: 720.66 g/mol, density: 1.65 g/ml). This non-polar molecule readily dissolves in non-polar solvents such as benzene, C6H6 (C6H6, molar mass: 78.11 g/mol, density: 0.8786 g/ml, Boiling pt: 80.10C, Freezing pt: 5.50C, kb = 2.53C/m, kf = 5.12 C/m). Consider a solution of C60 dissolved in benzene that is 15.60 % by mass and a density of 1.36 g/mL. What is the boiling point of this solution? ______________________________ over