Final Exam 2008
advertisement

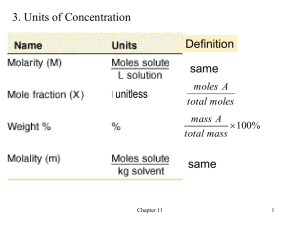
Types of Solutions Definitions Potpourri Reactions How Much? 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 In general, the symbol ‘aq’ next to a solute means that the solute has done this. What is dissolved in water? The ratio of moles of solute in solution to the total number of moles of solute and solvent. What is mole fraction? The concentration of a solution expressed in moles of solute per kg of solvent. What is molality? The solid produced in a double replacement reaction. What is a precipitate? [OH ] If a solution has of -4 5.82 x 10 , then the pH is this. What is 10.76? The calculation of the quantities of reactants and products involved in a chemical reaction What is stoichiometry? The reactant that gets completely consumed. What is the limiting reactant? The conditions of 0°C and 1 atm of pressure are more typically called this. What is STP (standard temperature and pressure)? Oil and water are called this because they are not soluble in each other. What is a immiscible? The two terms that specify what a soluble ionic compound and a soluble DAILY molecular compound, such as an acid, do when DOUBLE!!! dissolved in water, respectively. What are dissociate and ionize? 2Al2O3(s) 4Al(s) + 3O2(g) What is decomposition? C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) What is combustion? FeCl3(aq) + 3NaOH(aq) Fe(OH)3(s) + 3NaCl(aq) What is a double replacement reaction? The balanced equation and the states of the products of DAILY two reactants: Al(s) + Pb(NO3)2(aq) → DOUBLE!!! What is 2Al(s) + 3Pb(NO3)2(aq) → 2Al(NO3)3(aq) + 3Pb(s)? The net ionic equation of this reaction: 2Na3PO4(aq) + 3MnCl2(aq) Mn3(PO4)2(s) + 6NaCl(aq) What is 32+ 2PO4 (aq) + 3Mn (aq) Mn3(PO4)2(s)? The negative log of the hydroxide ion concentration in solution can be represented or stated as this. What is pOH? The formula of a substance written with the actual number of each element in one molecule of the compound. What is the molecular formula? This is produced when a substance accepts a hydrogen ion from another molecule. What is a conjugate acid? This happens if both products in a double replacement reaction are both soluble substances. What is nothing or no reaction? These are the products of a reaction between a strong acid and a strong base. What is a salt and water? The molar mass of CaO. What is 56.1 g/mol? The number of moles in 23 8.99 x 10 Ag atoms. What is 1.49 mol Ag? The freezing point of a 1.58 m NaCl aqueous solution. What is -5.88⁰C? A 0.550 M KBr solution is this concentration in %(m/v). What is 6.55%(m/v) KBr? The theoretical yield, in grams, of Ag2SO4 if 49.0 g of AgNO3 reacts with excess potassium sulfate. K2SO4(aq) + 2AgNO3(aq) Ag2SO4(s) + 2KNO3(aq) What is 45.0 g of Ag2SO4?