Physics 20 Name_________
advertisement

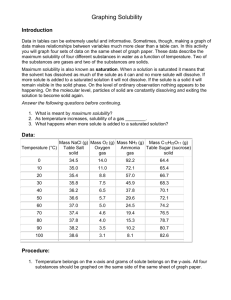
Chem 30 – Unit 2 Exam – Solutions and Solubility Name____________ March 4th, 2011 Part A. Match each of the statements from the first column with a term in the second column. Place the letter of the term in the second column in the correct blank. Each part is worth one mark. _____ the ability a substance has to dissolve at a specific temperature and pressure _____ least abundant substance in a solution _____ two or more substances mixed together forming a homogeneous mixture _____ act of adding more solvent to a solution _____ act of precipitating one ion at a time from a solution A. B. C. D. E. F. G. H. I. J. K. _____ most abundant substance in a solution L. _____ a solution containing the maximum amount of dissolved substance it can hold dilution concentration molarity precipitate saturated solution selective precipitation solubility solute solution solvent supersaturated solution unsaturated solution _____ a solution which can still dissolve more solute _____ a solid that forms when certain aqueous solutions are mixed together _____ a solution which contains undissolved solute Part B. Problems. Answer the following questions. Each question is worth a differing amount of marks. Questions out of 3 marks require the formula, substitution of values into the formula with correct units, and the correct answer with sig-figs and units. 1. What is the concentration of a solution of barium nitrate that contains 12.6 g of solute in 2.50 L of solution? 2. What volume of 12.0 M hydrochloric acid will contain 8.35 g? 3. If 0.200 g of sodium hydroxide are dissolved with water to form a 2.00 kg solution, what is the concentration of this solution in ppm? 4. What is the final concentration when 14.0 mL of a 4.20 M Na2CO3 solution is diluted to 86.0 mL. 5. If 200.0 mL of 0.200 M sodium sulfate is added to 800.0 mL of water, what is [Na+] and [SO42-]? 6. Describe how you would prepare 0.500 L of an 18.0 M solution of hydrobromic acid. 7. Using a solubility curve, answer the following questions: a) At what temperature will 75.0g of KNO3 completely dissolve in 100.0 g of water? Describe this solution in terms of saturation. b) How many grams of KClO3 will dissolve in 1.00 L of water at 70.0oC? Show your math. 8. For each of the following reactions predict the products with their states and balance the equation. If a reaction occurs write the balanced net ionic equation. If no reaction occurs, indicate so and state the spectator ions. (1 mark for each product with states, 1 mark for balancing the equation, 1 mark for balanced net ionic equation or stating no reaction with spectator ions) a) calcium carbonate and hydrobromic acid b) ammonium bromide and lead (II) acetate 9. Pb(NO3)2, Sr(NO3)2, and Mg(NO3)2 are all mixed together in an aqueous solution. Create a table to determine the order must you add NaOH, HCl, and (NH4)2SO4 to selectively precipitate each original cation out individually. Which precipitates will form with each solution added? Order Precipitate