benzene manufacturing
advertisement

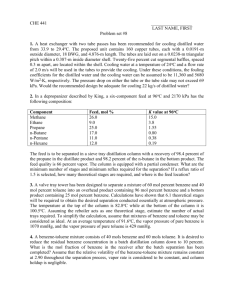
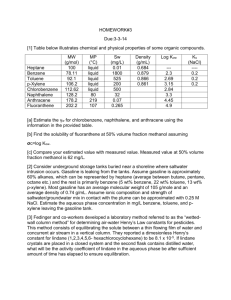
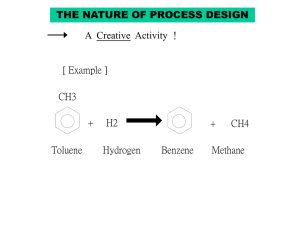
PONNALA SWATHI-B130896CH RAHUL CR-B130746CH REGHURAM V-B130245CH CONTENTS Raw Materials Chemical Reactions Properties Flow Chart Process descriptions Engineering Problems Alternate Process for Productions RAW MATERIALS (HYDRODEALKYLATION ) Hydrodealkylation is the named process for the conversion of toluene to benzene through reaction with hydrogen gas . The principal raw materials for the process are obviously: TOLUENE HYDROGEN Note: The above process can be used for preparing naphtha from alkyl naphthalenes. CHEMICAL REACTIONS Toluene adds hydrogen gas to produce benzene and methane. 2C6H5CH3 + H2 --------------> C6H6 + CH4 Dimethyl benzenes undergo similar reactions with 2 moles of hydrogen. C6H4(CH3)2 + 2H2-------------> C6H6+2CH4 Properties of benzene Molar mass : 78.11g/mole Appearance: It is a colourless liquid with an odour similar to that of most gasoline compounds. Density: 0.8765 g/ cu.cm Melting point: 278.68K Boiling point:353.2K Solubility: Soluble in chloroform, alcohols and organic solvents. Flow chart: PROCESS DESCRIPTION: Hydrodealkylation of toluene can be operated under catalytic or thermal conditions. Catalytic process requires milder conditions of 600-650oC and lower pressures whereas the thermal process requires temperatures above 800oC. Toluene is mixed with heavier aromatics or paraffins from the benzene fractionation column. The make up gas rich in hydrogen is compressed with recycle gas and mixed with the liquid toluene stream. The combined feed is then preheated using heat exchange and then into a fired preheater before being charged into the reactor. The reactor effluents are cooled by heat exchange and then by water before being sent to a separator. The liquid and gas components are separated here. The gas containing methane and remaining hydrogen is either used directly as fuel or as a source of methane. The liquid is then sent to a stripping column where residual gases are also removed. The gas free liquid is then sent into the fractionator where benzene is separated out. Engineering Problems: Reactor design: Catalytic processes were first utilised first ,operating at 600-650oC and pressure 35-40 atms. Economic balances in yield include residence time,temperature and reaction rates.Comparitive yields were obtained at higher temperatures and pressures. Hydrogen problems: The use of hydrogen requires explosion-proof plant construction and its use at high reaction conditions need use of chrome steel to avoid embrittlement. Other processes Catalytic Reforming: Catalytic reforming involves the dehydrogenation of naphthenes to aromatics, or the isomerization of alkyl naphthenes and the dehydrogenation of them. Toluene disproportionation: Alkylated aromatics are trans alkylated to produce benzene and alkylated benzenes. Pyrolysis gasoline distillation to remove benzene and diolefins. USES OF BENZENE Benzene is mainly used as intermediate to make their chemicals, most important of which include cumene, ethyl benzene and cyclohexane. It finds extensive use in producing precursors for plastics, resins, rubber, pesticides, explosive lubricant, detergents and fibres. EXPOSURE AND HEALTH HAZARDS • Benzene increases the risk of cancer and other illnesses, also causing bone marrow failure ,aplastic anaemia, acute leukaemia and related diseases in substantial amounts. • It targets liver, kidney,lung,heart and the brain and can cause DNA strand breaks. Vapours from products that contain benzene such as glues,paints,furniture,detergents etc. can also be a source of exposure. Exposure of the general population to benzene occurs while breathing ,the major sources being tobacco smoke and automobile service stations . Certain batches of soft drinks manufactured across various parts of the world have been found to contain benzene levels above the limits prescribed by the world health organisation. THANK YOU