a561c16123e6e7f
advertisement

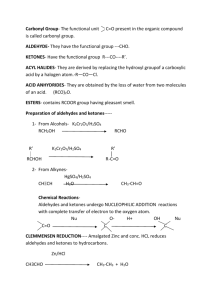
ALDEHYDES & KETONES (ALKANALS & ALKANONES) 1 alkane alcohol reduction reduction aldehyde ketone oxidation carboxylic acid addition product nucleophilic addition Aldehydes and ketones are characterized by the the carbonyl functional group (C=O). Some common classes of carbonyl compounds Carbon is sp2 hybridized. C=O bond is shorter, stronger, and more polar than C=C bond in alkenes. Aldehydes are named by replacing the terminal -e of the corresponding alkane name with –al The parent chain must contain the CHO group The CHO carbon is numbered possible minimum number. Replace the terminal -e of the alkane name with – one Parent chain is the longest one that contains the ketone group Numbering begins at the end nearer the carbonyl carbon Good solvent for alcohols. Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O-H or N-H. Acetone and acetaldehyde are miscible in water. Preparation of Aldehydes & ketones 1] Oxidation of 1& 2 alcohol : RCH2OH C2H5OH O R-C-H + H2O CrO3 / pyridine or, Cu /heat(week oxidi). [O] R R-C-OH H CH3CHOHCH3 isopropanol CH3CHO [O] CH3COOH KMnO4 neutral [O] CH3COCH3 O R-C-R More polar, so boiling point higher than corresponding alkane or ether. Absence of H-bond, so boiling point lower than corresponding alcohol. A- 10 Alcohol gives aldehydes: Cu/ 350 oC CH3CH2OH CH3CHO + H2 B- Secondary Alcohol gives Ketones: OH O Cu/350oC CH3CHCH3 CH3CCH3 + H2 3] Ozonolysis of alkenes: CH3 C=CH2 CH3 1) O3 2)Zn, H2O CH3 C=O CH3 CH2O H 2O/H 2SO 4 R C CH R C CH 2 HgSO 4 OH R C CH 1. 9-BBN 2. H 2O 2/OH - RCH CH OH R C CH 3 O R CH 2 C H O A) If dihalogen are terminal: H2O/NaOH H2O CH3CHCl2 CH3CH(OH)2 CH3CHO acetaldehyde B) If dihalogen aren’t terminal: H2O/NaOH CH3CCl2CH3 CH3COCH3 acetone R-CO-Cl CH3COCl H2/Pd H2/Pd R-CHO +HCl CH3CHO + HCl 1] Reactions with Grignard reagent: R-CHO + R'-MgX 1)dry ether 2) H3O R-CHOH-R' 1)dry ether CH3-CHO + CH3-MgCl 2) H3O R-CO-R' +R''-MgX 1)dry ether 2)H2O CH3CO-CH3 +CH3-MgX CH3CHOHCH3 R'' R-C-R' OH 1)dry ether 2)H2O OH CH3-C-CH3 CH3 Nucleophilic addition to carbonyl: O C + Y Z OY C Z In presence of dry HCl aldehydes and ketones react with two equivalent of alcohols to form acetals and ketals Acetal Formation Acetals are geminal diethers- structurally related to hydrates, which are geminal diols. R O C R O C + R + R'OH H - R'OH aldehyde R O C + R'OH R - R'OH ketone - OH R C OH R H2O H2O OH H C OR' R + R'OH OH R C OR' R + R'OH hydrate (gem-diol) OR' H C OR' + R - R'OH acetal hemi-acetal (gem-diether) - R'OH hemi-ketal OR' R C OR' R H2O + H2O ketal (gem-diether) 19 4-Reduction of Aldehydes/Ketones Hydrogenation H2 R C H Pt O RCH 2OH Primary ROH H2 R C R' Pt O H R C R' OH Secondary ROH 1- Hydroxylamine: -C=O + NH2OH -C=NOH + H2O (oxime) CH3COCH3 + NH2OH 2- Hydrazine: -C=O + H2N-NH2 CH3CHO +H2N-NH2 CH3-C=NOH CH3 -C=N-NH2 + H2O (hydrazone) CH3CH=N.NH2+H2O Aldol Condensation - Under the influence of dilute base or dilute acid two molecules of an aldehyde or a ketone may combine to form bhydroxaldehyde or b-hydroxyketone. This reaction is called aldol condensation. The Aldol Condensation ald + ol O R CH2 C H OH + base O R CH2 C CH C H O H R an aldol (b-hydroxyaldehyde) R CH2 C H H3O+ - H2 O O aldols easily lose water to form a double bond R CH2 CH C C H R a,b-unsaturated aldehyde Aldol Condensation -- Mechanism : O: _ .. + :O .. H CH3 C H fast : O: slow CH3 C H _ .. CH2 : O: .. _ : O: CH3 C CH2 C H H + H2O : O: _ .. CH2 C H fast : O: .. :O H forms new C-C bond : O: CH3 C CH2 C H C H enolate ion .. _ : O: CH3 C H H2C CH2 C H C H : O: .. _ :O: H _ .. + :OH .. 1- CH3COCH3 3I 2 Cl3COCH3 2- Cannizzaro’s reaction: 2CH2O + NaOH NaOH CHI3 + CH3CO2Na CH3OH + HCOONa Cannizzaro’s reaction Add ammonia solution to AgNO3 solution until precipitate dissolves. Aldehyde reaction forms a silver mirror. O R C H + 2 + H3)2 _ + 3 OH + Ag(NH3)2 O _ + H2O 3 OH O H2O 2 Ag + R C O 2 Ag + R C O _ + _ + 4 NH3 + 2 H2O 4 N KMNO 4 RCHO RCOOH or K 2Cr2O7 , H Tollen’s test RCHO 2Ag NH3 2 3OH RCOO 2Ag 4NH3 2H2O (Silver mirror) (ammonical silver nitrate solution) Fehling’s test RCHO 2CuO RCOOH Blue Schiff’s test Cu2O Re d ppt. (Cuprousoxide) Schiff's Test for aldehydes. Use 2 mL Schiff's reagent + 3 drops unknown. Positive test showing a magenta color after ten minutes. A carboxylic acid Contains a carboxyl group, which is a carbonyl group (C=O) attached to a hydroxyl group (— OH). Has the carboxyl group on carbon 1. carbonyl group O CH3 — C—OH hydroxyl group or CH3COOH carboxyl group 29 The IUPAC names of carboxylic acids Replace the -e in the alkane name with -oic acid. CH4 methane CH3—CH3 ethane HCOOH methanoic acid CH3—COOH ethanoic acid Number substituents from the carboxyl carbon 1. CH3 O | ║ CH3—CH—CH2—C—OH 4 3 2 1 3-methylbutanoic acid 30 Carboxylic acid R-COOH Aliphatic (carboxylic cid) Ar-COOH aromatic (benzoic acid) Nomenclature : 1) replace ane by -ic acid No. C Formula IUPAC Common 1 HCOOH Methanoic acid Formic acid 2 CH3COOH Ethanoic acid Acetic acid 3 CH3CH2COOH Prpanoic acid Prpionic acid 4 CH3(CH2)2COOH Butanoic acid Butyric acid 5 CH3(CH2)3COOH Pentanoc acid valeric acid 2) Longest continuous chain CH3CH2CHCH2CH2COOH 4-Methyl hexanoic acid CH3 ba C-C-C-C-C-COOH CH3CH2CHCH2CH2COOH CH3 γ-Methyl hexenoic acid CH3 CH3 CH3-CH-CH-COOH commmone: IUPAC: a-b- Dimethyl butyric acid 2,3-Dimethyl butanoic a CH3-CHBr-CHCl-CO2H 3-Bromo-2-chlorobutnoic acid Methanoic acid (formic acid) O ║ H─C─OH ethanoic acid (acetic acid) O ║ CH3─C─OH 33 Physical properties: 1] They form hydrogen 2] comp. 1-7 soli in H2O . 3] mor than 7 carbon less soli. (bec. R increased) 4] Aromatic acids insoluble. In H2O 5] BP. Acid > Alcohol Carboxylic acids Are strongly polar. Have two polar groups: hydroxyl (−OH) and carbonyl (C=O). δO ║δ+ δ- δ+ CH3CO H 35 The boiling points of carboxylic acids Are higher than alcohols, ketones, and aldehydes of similar mass. Are high because they form dimers in which hydrogen bonds form between the polar groups in the two carboxyl groups. O H—O || | CH3—C C—CH3 | || O—H O A dimer of acetic acid 36 Carboxylic acids Form hydrogen bonds with many water molecules. Water molecules With 1-4 carbon atoms are very soluble in water. 37 Preparation of carboxylic acid 1] Oxidation a) 1 alcohols & Aldehydes CH3CH2OH KMnO4 / H+ CH2OH KMnO4 / H+ or K2Cr2O7 /H+ CH3CHO heat CH3COOH COOH CHO heat Cu / Heat or CrO3 / pyridine R-CHO RCH2OH K2Cr2O7 / H+ or KMnO4 / H+ RCOOH Carboxylic acids can be prepared by oxidizing primary alcohols or aldehydes. The oxidation of ethanol produces ethanoic acid (acetic acid). OH O | [O] || CH3—CH2 CH3—C—H O [O] || CH3—C—OH ethanol ethanal ethanoic acid (ethyl alcohol) (acetaldehyde) (acetic acid) 39 2] Carbonation of Grignard reagent: R-Mg X 1) CO2 R-COOMgX 1)CO2 CH3-Mg Br 2) H3O RCOOH 2) H3O CH COOH 3 CH3-COOMgBr + Mg(OH)Br RMgX + O C O O R C O- H+ + +MgX O R C OH Reactions of acids 1)Salt formation: it react with strong base & we can use Ca or K RCOOH + KOH RCOOH + NaOH It reacts with weak base RCOOH + NaHCO3 RCOOK + H2O RCOONa + H2O sodi. acetate RCOONa + CO2 + H2O Sodium bicarb. Can be used to distinguish between carboxylic acid and phenols OH + NaHCO3 NO reaction 2) Formation of Ester: nucleophilic substitution R C O + H O R OH carboxylic acid alcohol H+ R C O + H2O O R ester condensation reaction reverse = hydrolysis ester + H2O H+ carboxylic acid+ alcohol 2) Formation of Ester: RCOOH R'-OH / H+ heat OH COOH R-COO-R' + H2O OH COOC2H5 CH3CH2OH H+ / heat 3) Formation of acid chloride: 2 RCOOH 2 CH3COOH SOCl3 , PCl3 or PCl5 2 SOCl3 , PCl3 or PCl5 O R-C-Cl 2 O CH3-C-Cl 4) Formation of acid anhydride: 2RCOOH + P2O5 (RCO)2O + H2O 2CH3COOH + P2O5 (CH3CO)2O + H2O 5- Reduction: RCO2H + LiAlH4; then H+ RCH2OH LiAlH4 1o alcohol H+ CH3CH2CH2CH2CH2CH2CH2COOH Octanoic acid (Caprylic acid) CH3CH2CH2CH2CH2CH2CH2CH2OH 1-Octanol 6- Decarboxylation:( Soda lime) CH3COOH + NaOH/CaO CH4 + Na2CO3 Alkane ketone aldehyde ROR ROH RCOOH alkene Alcohols are central to organic syntheses RX RH alkyne