electron configuration
advertisement

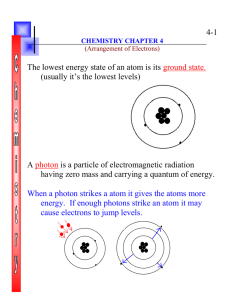
Faculty of Allied Medical Sciences General Chemistry (MGGC-101)& (GRGC-101) Electron Configuration and periodicity Supervision: Prof.Dr.Shehata El-Sewedy Dr.Fatma Ahmed Outcomes By the end of this lecture, the students will be able to 1-Understand the electron configuration 2-learn to rules used to build the electron configuration in many-electron atoms 3- To know valence shell electrons 4-Recognize Pauli exclusion principle An “electron configuration” of an atom is a particular distribution of electrons among available subshells. Orbital diagram: a diagram used to show how the orbitals of a subshell are occupied by electrons. Each orbital is represented by a box and each group of orbitals is labeled by its subshell notation. Electrons are represented by arrows: up for ms = +1/2 and down for ms = -1/2. Example So the orbital diagram for Hydrogen (Z=1) is as follows: 1S It is convenient to have a shorthand notation for representing the electron configuration. This is done by writing the symbol of each orbital occupied by an electron with a superscript to indicate the number of electrons occupying that orbital. Thus, for hydrogen, the electron configuration is : denotes the numer of electrons in the orbital or subshell denotes the principle quantum number n 1 1S denotes the angular quantum number l Rules used to build the electron configuration in many-electron atoms Three rules govern the arrangement of electrons in atoms: 1-The Aufbau Principle - (means building-up in German): Electrons are added to sublevels of lower energy then to the higher one 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f 4s<3d in energy so 4s filled first You need not memorize this order. As you will see, it can be easily obtained: Order for Filling Atomic Subshells 2-Hund’s rule states degenerated orbitals (orbitals of same energy) are filled singly with electrons first (unpaired) with parallel spin rather than pairing to avoid repulsion between electrons Ex P3 orbital Un stable Un stable Stable 3-Order of stability Completely filled orbital > half filled orbital More stable > partially filled orbital less stable 4-Pauli exclusion principle states that no two electrons in the same atom have the same values of all four quantum no.∴ an orbital will have maximum of 2 electrons. ↑ unpaired electron ↑↓ paired electrons and not ↑↑ or ↓↓. Ex (N=2) 1S2 For first electron n=1, l=0, m=0, S= + For second electron n=1, l=0, m=0, S= - 5-Valence shell electrons (outer most shell) It’s the electrons in the last shell in the atom Ex: 6-Electronic configuration according to nobel gases (inert gases) Ex Write the electronic configuration of 29Cu and 24Cr comment in your answer? Answer 29Cu (At.no.= 29) 29Cu 1S2 2S2 2P6 3S2 3P6 4S2 3d9 Less stable xxxxx So One electron from 4S2 migrates to 3d9 to be completely filled 3d10 which is more stable Then 29Cu 1S2 2S2 2P6 3S2 3P6 4S1 3d10 more stable Completely filled >> partially filled 1 10 29Cu 18[Ar] 4S 3d √√√√√ 24Cr 1S2 2S2 2P6 3S2 3P6 4S2 3d4 24Cr 1S2 2S2 2P6 3S2 3P6 4S1 3d5 half filled >> partially filled 1 5 24Cr 18[Ar] 4S 3d Less stable more stable xxxxx √√√√√ Write the electronic configuration of O+, O, O-, O-2 & what are the valence shell electrons of O? Answer 8O = O-e- = 8-1= 7 e-s 8O = O+e = 8+1= 9 e s O-2 v.s.e of oxygen 8O are 6 e-s 8O + O+ 1S2 2S2 2P3 O- 1S2 2S2 2P5 1S2 2S2 2P6 Quiz time Write the electronic configuration of 29Cu and 24Cr comment in your answer? Student Question Define and give example Valence shell electrons Hund’s rule states Write the electronic configuration of 29Cu and 24Cr comment in your answer? Write the electronic configuration of O+, O, O-, O-2 & what are the valence shell electrons of O? Assignments Group A and B Homogenous mixture in industrial world Moemen Mohamad El-said Abeer Salah Farida Maher Molecular Formula Ahmad Rada Kamel – Ahmad Mohamad Abd El SalamAhmad El Said- Ahmad Alaa Elemental analysis Sara Lotfy- Shimaa Saied – Esraa Sameh-Ahmad Shadra-Ibrahim ElHabet- Ibrahim El Said Physical properties in Chemistry Mohamad Ramadan - Mohamad Nabil- Mahmoud Hamdi- Abd El Rahman Abd El-Moneim –Yossef Gamal- Mohamad Shabaan- RECOMMENDED TEXTBOOKS: 1-Raymond Chang. Chemistry. 10th ed. 2009 2-Zumdehl. International edition. 2009