CHEM 5013
advertisement

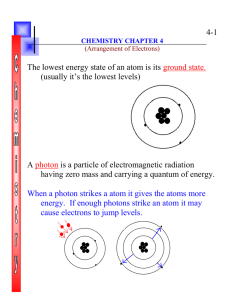
CHEM 5013 - Applied Chemical Principles Suggested problems for Quiz #8 on Thursday November 14th 1. Explain in your own words the processes of absorption and emission of light by an atom. That is, what is happening at the atomic level? Absorption – Electron absorbs energy from an incident photon and this pushes the electrons to excited (higher energy) states Emission – The electrons return to ground state (lowest energy level) and while doing so, emit an amount of energy equal to the amount absorbed. This energy is seen as light 2. What is the wavelength of microwave radiation whose frequency is 1.315x10 9 Hz? Report your answer in nanometers. λ = c/v = 3 x 108 m/s 1.315 x 109 /s = 0.228 m x 1 nm 1 x 10-9 m = 2.28 x 108 nm 3. What is the energy (in Joules) of a photon corresponding to the microwave radiation in question #2? E = hv = (6.63 x 10-34 J▪s) ( 1.315 x 109 Hz) = 8.72 x 10-25 J 4. Mercury vapor lamps are increasingly being phased out in the United States. One wavelength of emission provided by a mercury vapor lamp is known as the G-Line (the violet colored line) and has a wavelength of 435.8 nm. Calculate the energy in a single photon of light emitted at the G-line of a mercury vapor lamp. 435.8 nm x (1x10-9m/1 nm) = 4.358 x 10-7 m E = h c / λ = (6.63 x 10-34 J▪s) (3 x 108 m/s) = 4.56 x 10-19 J 4.358 x 10-7 m 5. Provide the following: a. The full ground state electron configuration for an atom of Tin 1s22s22p63s23p64s23d104p65s24d105p2 (50 total electrons) b. The orbital diagram for an atom of Iron 1s2 2s2 2p6 3s2 3p6 4s2 3d10 6. Give two different possible orbital diagrams for the 1s22s22p4 configuration of an atom of oxygen, one of which corresponds to the ground state. 1. GROUND STATE ORBITAL DIAGRAM 2. EXCITED STATE ORBITAL DIAGRAM ANYTHING ELSE 1s2 2s2 2p4 7. Which of the following possible orbital diagrams are allowed and which are not according to the Pauli Exclusion Principle? For those that are not allowed, explain why they are not allowed. (1 pt each) a. b. 1s 2s 2p 1s ALLOWED – Just not ground state c. 2s 2p NOT ALLOWED – only allowed to have 2 electrons in 2s orbital d. 1s 2s 2p NOT ALLOWED – same spin on 2 Electrons in 1s orbital 1s 2s 2p ALLOWED – Just not ground state 8. Which of the following electron configurations is possible? For those that are not possible, explain why they are not possible. a. 1s22s12p6 b. 1s22s22p63s23p63d7 Possible but not Ground State c. 1s22s22p8 Not possible – can only have 6 electrons in a p subshell Possible d. 1s22s32p63s13d9 Not possible – can only have 2 electrons in s subshell