Exothermic and Endothermic Reactions
advertisement

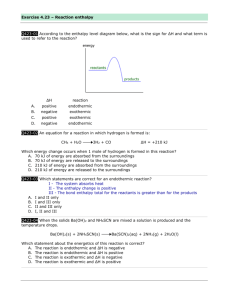
Chapter: 5 Energetics and Thermochemistry Title: Lesson 1 Exothermic and Endothermic Learning Objectives: – Reflect on prior knowledge of the energy changes in chemical reactions – Understand the difference between endothermic and exothermic reactions – Draw enthalpy level diagrams showing the relative stabilities of products and reactants – Complete an experiment investigating endothermic and exothermic reactions Energy and Heat Transfer Energy Energy is a measure of the ability to do work. We will be focusing on reactions involving heat changes. Heat is a mode of energy transfer which occurs as a result of a temperature difference. Heat increases the average kinetic energy of the molecules in a disordered fashion. However, work can be contrasted as being a more ordered process of transferring energy. Work on lifting a beaker of water lifts all the water molecules in the same way! Main Menu System and Surroundings When chemical changes happen it is useful to distinguish between the system (area of interest) and the surroundings (everything else!) Most reactions take place in an open system. Energy and matter can be exchanged with the surroundings. A closed system can exchange energy but matter cannot be exchanged with the surroundings. Total energy cannot change during the process. Any energy lost by the system is gained by the surroundings and vice versa. Main Menu Enthalpy, H This is a measure of the energy locked up inside chemicals We can only measure changes in enthalpy, ∆H, not enthalpy itself Substances with lower enthalpy are more stable than those with higher enthalpy Enthalpy of reactants and products are not the same (energy is either taken in or given out during the reaction) Enthalpy level diagrams show the changes in enthalpy over the course of a reaction. ∆H can be observed as change in temperature (measured or calculated) Main Menu ∆H is positive when heat is added to the system ∆H is negative when heat is released from the system into the surroundings Main Menu ENDOTHERMIC & EXOTHERMIC Chemical reactions can either release energy to their surroundings, EXOTHERMIC __________, or energy can be transferred to them from the surroundings, http://www.youtube.com/watch?v=pENDOTHERMIC ____________. 27I_osoaw KEY IDEA •Bonds contain enthalpy. •Energy is absorbed to break bonds apart in the REACTANTS in a chemical reaction. •Energy is released when new bonds form in the PRODUCTS in a chemical reaction. •When bonds break, energy is absorbed (endothermic – get cooler) . Self sustaining until reactants run out. •When bonds form, energy is released (exothermic – get hotter). Needs energy for the reaction to keep going or runs out when reactants run out. ENDOTHERMIC & EXOTHERMIC EXAMPLES: Exothermic: A reaction endothermic in one direction is always exothermic in the other • Neutralising an acid with an alkali • Burning magnesium • Adding water to anhydrous copper sulphate Endothermic: • Thermal decomposition of limestone • Photosynthesis • Heating hydrated copper sulphate BBC Bitesize Hydrated Copper Sulphate Explanation Good examples of exothermic and endothermic reactions used in everyday life are the HOT PACKS and COLD PACKS used for treatment of sprains and other muscular disorders. HOT PACKS COLD PACKS A hot pack is a plastic bag filled with saturated sodium ethanoate (CH3COONa). A typical cold pack contains the ionic compound ammonium nitrate (NH4NO3) and water. It also includes a small concave metal disc. When allowed to mix, the nitrate goes into solution and the electrostatic forces of attraction between the ammonium ions (NH4+) and nitrate ions (NO3-) are broken. Twisting the disc causes “nucleation “ (first phase of crystallisation) and the sodium acetate begins to crystallize very rapidly. Since bonds are being formed, energy is released and the pack becomes hot. The hot pack is 'recharged' by boiling it in water for several minutes. Energy is taken in from the surroundings to overcome these forces, thus causing the cooling effect. ENTHALPY CHANGES ENTHALPY (H) = the energy content of a substance measured at constant pressure. H = enthalpy change = enthalpy of PRODUCTS – enthalpy of REACTANTS Enthalpy (H) Products (P) A Reactants (R) H +ve H -ve B For reaction A Reaction Path (ie time) For reaction B H is POSITIVE H is NEGATIVE ENDOTHERMIC reaction EXOTHERMIC reaction heat energy ABSORBED heat energy RELEASED net bond BREAKING net bond FORMATION MgCO3(s) MgO(s) + CO2(g) CH4(g) + 2O2(g) CO2(g) + 2H2O(g) The units are kilojoules per mole (kJmol-1) ENTHALPY Enthalpy Level Diagrams Endothermic Exothermic Heat taken in Energy level of products is higher than reactants so heat taken in from surroundings. Heat given out Energy level of products is lower than reactants so heat given out to surroundings. EXOTHERMIC An exothermic enthalpy change is always given a negative value, as energy is lost to the surroundings. ΔH = -kJmol-1 ΔH = ΔH products - ΔH reactants Small number Bigger number ENDOTHERMIC An endothermic enthalpy change is always given a positive value, as the energy is gained by the system from the surroundings. ΔH = + kJmol-1. ΔH = ΔH products - ΔH reactants Big number Silly Video Small number Enthalpy level diagrams These show the changes in enthalpy over the course of a reaction H H ∆H is negative (H goes down) ∆H is positive (H goes up) Activation energy small Activation energy large Products more stable Products less stable Break weaker bonds, make stronger bonds Break stronger bonds, make weaker bonds Chemical energy turned into heat energy Heat energy turned into chemical energy Main Menu Simplified diagrams These are the level needed by the IB Less useful as they tell you nothing about activation energy and so on Main Menu Intermediates Some reactions form intermediate products A product that quickly turns into something else This can lead to more complicated enthalpy level diagrams Main Menu STANDARD ENTHALPY CHANGES Standard conditions: •Pressure 100kPa •Concentration of 1 mol dm-3 for all solutions •All substances in their standard states •Temp 298K •ΔHθ ‘delta H standard’ ENDOTHERMIC & EXOTHERMIC QUANTITIES: • Energy is measured in kilojoules per mole • kJmol-1 CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) • Combustion of methane one mole of methane reacts with 2 moles of oxygen • It gives out 890kJ of energy ENTHALPY Question ENTHALPY Answer ENTHALPY PHYSICAL STATES Whether products and reactants are solids, liquids or gases affects the enthalpy change of a reaction. Heat is put in to change a liquid to a gas (endo) Heat is given out when a gas turns to a liquid (exo) H2 (g) + ½O2 (g) H2O (l) ΔH = -285.8kJmol-1 H2 (g) + ½O2 (g) H2O (g) ΔH = -241.8kJmol-1 Difference in the ΔH’s represents the amount of heat needed to turn one mole of water into 1 mole of steam ENTHALPY 1.Balance each of the equations below 2.Name the compounds or elements present in each one 3.Identify each one as exothermic or endothermic. 4.Draw the energy level diagram for each CH4(s) + O2(g) → CO2(g) + H2O(l) ΔH = -890 kJmol-1 HCl(g) → H2(g) + Cl2(g) ΔH = 185 kJmol-1 NH3(g) + O2(g) → NO(g) + H2O(l) ΔH = -1169 kJmol-1 ENTHALPY ANSWERS CH4(s) + 2O2(g) → CO2(g) + 2H2O(l) Methane Oxygen Carbon dioxide Water ENDOTHERMIC 2HCl(g) → H2(g) + Cl2(g) Hydrogen chloride Hydrogen Chlorine 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l) Ammonia Oxygen EXOTHERMIC Nitrogen monoxide EXOTHERMIC Water For each of the reactions above, construct a simple enthalpy level diagram showing the enthalpy change. CH4(s) + 2O2(g) ΔH =-890 kJmol-1 CO2(g) + 2H2O(l) H2(g) + Cl2(g) ΔH = +185 kJmol-1 2HCl(g) 4NH3(g) + 5O2(g) ΔH =-1169 kJmol-1 4NO(g) + 6H2O(l) ENTHALPY PLENARY Cut out the boxes and stick them in the right place to summarise enthalpy changes for exo and endothermic reactions Key Points Exothermic reactions Endothermic reactions Give out energy Absorb energy Decrease in enthalpy Increase in enthalpy Products more stable Reactants more stable Main Menu