Pre-Lab Part I (18 pt)
advertisement

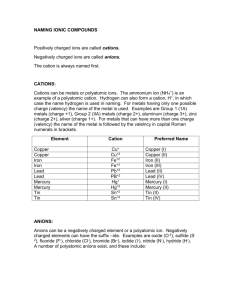
YOUR NAME HERE, YOUR CLASS PERIOD HERE (2 pt) Solubility Rules Lab Introduction: Anions can be grouped by their solubility: some are usually soluble and some are usually insoluble. However, the cation that is paired with these anions affects the solubility. In this lab you will be investigating three anions that are usually insoluble, and developing rules for which types of cations are exceptions to this rule. Pre-Lab Part I (18 pt) Describe the differences between a cation and an anion below: (6 pt) Type of Ion Charge? Where found in formula? Examples Cation Anion Identify the anion and cation in each of the following examples: (12 pt) Compound KBr Cation Anion Compound NH4F CaCl2 Sr3(PO4)2 Cs2CO3 NH4CO3 Cation Anion Pre-Lab Part II: (15 pt) 1. A precipitate is a _________ that is produced from a reaction of ___________ solutions. 2. Identify the precipitate in the following reaction: AgNO3(aq) + NaCl(aq) NaNO3(aq) + AgCl(s) 3. When a soluble substance is placed in water it will _____________ and the particles will form a ______________ mixture with the water. 4. When an insoluble substance is placed in water it will not ___________ and will either __________ or ________ in the container. 5. Suppose two solutions, when mixed together, undergo a chemical reaction. If one of the products is insoluble, you would expect to see the insoluble product as a ______________ that will make the solution ____________. 6. The alkali metals are Li, _____, _____, _____, _____, and Fr. 7. The type of ion that alkali metals form is __________. YOUR NAME HERE, YOUR CLASS PERIOD HERE (2 pt) Procedure and Data: (15 pt) 1. On a white paper, place your well plate, and label it with the chemicals on the “well plate” pictured below. 2. In the first well of a spot plate, combine 6 drops of the reactant from column 1 with 6 drops of the reactant from row 1. 3. Repeat steps 1 and 2 for all remaining column and row combinations. **Some reactions take some time. Wait a few minutes before completing step 4. ** 4. In the corresponding box below, write P if precipitate formed or write NR for no reaction. Anions→ Cations↓ NH4Cl Na2CO3 NaOH Na3PO4 Anion?______ Anion?______ Anion?______ . . . . . . . . . . . . Cation?_____ FeCl3 Cation?______ MgCl2 Cation?______ KCl Cation?______ Analysis: Answer all questions in complete sentences, except for where it says “List”. 1. Consider the cations you used in the reactions above: (6 pt) a. List every cation here: (there should be five!) b. List the cation(s) that usually react with another chemical to form soluble products. c. List the cation(s) that usually react with another chemical to form insoluble products. d. What conclusion can you draw about alkali metal cations? e. Does NH4+ react to form a precipitate with any of the anions we tested? f. What does that suggest about the solubility of the NH4+ cation? YOUR NAME HERE, YOUR CLASS PERIOD HERE (2 pt) 2. Consider the anions you used in the reactions above: (4 pt) a. List every anion here: (there should be four!) b. With what cation(s) will CO32-, OH-, and PO43- all react to create a precipitate? 3. Do your results match what we know from the solubility chart? Use examples from the lab to explain why or why not. (5 pt) 4. Now, practice using the solubility chart on your periodic table and answer the following. (10 pt) Compound Solid or aqueous? Compound Solid or aqueous? KBr AgBr Mg(OH)2 LiOH Cs2SO4 BaSO4 CaCO3 PbCO3 NH4F (NH4)3PO4 Conclusion: A Paragraph of Awesomeness (30 pt) 1. Paragraph: must be written as such (2 pt) 2. Spelling and grammar (3 pt) 3. Complete sentences. (5 pt) Write an expository paragraph using complete sentences and your own words. Include the information from each bullet point below. Include the following information in your paragraph. Use the answers to the analysis questions to help you: ● Purpose of the lab ● Procedure ● Explanation of your cation results ● Explanation of your anion results ● Summarize: what did the results of this lab demonstrate about solubility rules? You must correctly use the following ten vocabulary terms (10 pt): Soluble, insoluble, precipitate, cation, anion, well plate, aqueous, solution, reactant, product. **UNDERLINE when they appear in your paragraph!! WRITE YOUR PARAGRAPH BELOW USING COMPLETE SENTENCES, INCLUDING ALL BULLET POINTS, AND INCLUDING ALL VOCABULARY TERMS, UNDERLINED.