Ionic Bonding Puzzle Lab Worksheet
advertisement

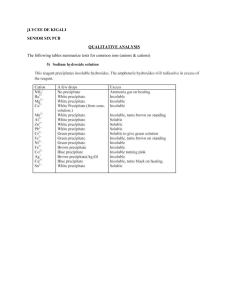
Ionic Bonding Puzzle Lab Name __________________________________________ Block _____ In this activity you will create models of ionic compounds and observe the chemical formula of the binary compounds you have created. Binary compounds are made of only two types of ions. Cations are positively charged, and anions are negatively charged. Because opposite charges attract, the cations and anions forge ionic bonds between each other. PRE-LAB QUESTIONS: 1. Use the word bank to complete the following statements (each term can be used more than once). anions formula lose cations gain neutral charges ions subscripts a. ___________________ are charged particles that form when electrons are transferred between atoms. b. Metals ___________________ electrons and become positive ions known as ___________________. c. Non-metals ___________________ electrons and become negative ions known as ___________________. d. The ___________________ show the number of each atom in a chemical ___________________. e. For an ionic bond, the ___________________ need to balance. f. The chemical___________________ shows how many of each ion are needed to balance the ___________________ and make the compound ___________________. 2. Observe the puzzle pieces and notice the shape and charge of each cation model. What do you think each triangle indentation for the cations represents? 3. Observe the puzzle pieces and notice the shape and charge of each anion model. What do you think each triangle tab for the anions represents? 4. Based on this model, what shape to you think a neutral atom would be for these elements? PROCEDURE: 1. Organize the ion models by their groups on the periodic table. a. What do you notice about the number of triangular indentations or tabs on each model? b. Is there a connection between the number of triangular indentations or tabs on each model and its charge? c. Is there a pattern to the charge of the ions from the same group on the periodic table? 2. Complete the following table using the ions indicated. Record the cation, anion, chemical formula, and chemical name for each compound you create. Chemical names are written with the cation name first followed by the anion name. You cannot repeat compounds that you create. Cation Anion P3P3N3N3O2O2S2S2- Al3+ Al3+ Al3+ Fe3+ Fe3+ Fe3+ Li1+ Na1+ K1+ Fe2+ Fe2+ Sr2+ Sr2+ Rb1+ Ti4+ Ti4+ Chemical Formula Chemical Name 3. In the compounds you formed, what type of element (metal, nonmetal, metalloid) are the cations? What type of element are the anions? cation: anion: 4. What is the overall charge of each ionic compound that you formed? 5. Observe the names of the ions on the cards. a. How do the names for the cations compare to the name of the neutral atom or element? b. How do the names for the anions compare to the name of the neutral atom or element? c. Do all metals have a number in parenthesis? If not, what group(s) from the periodic table have parenthesis? 6. Using the periodic table of elements, draw the shape of what a model of the compound gold (III) selenide would look like. Be sure to include the ion and its name on each piece that you draw. Finally, write the chemical formula for gold (III) selenide. Chemical Formula: ________________________