Atomic Structure and Nuclear Chemistry
advertisement

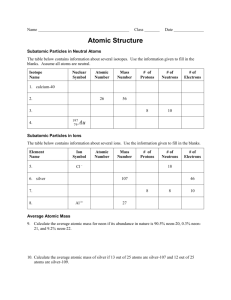
Atomic Structure and Nuclear Chemistry Chapter 4 and 18 Elements a.k.a atoms Robert Boyle first defined an element as a substance which could no longer be broken down into other substances Each element has unique properties Many early scientists speculated how the element or atom was structured Theory of atomic structure evolved from early thoughts to today’s atom What do you know about the atom? Take a moment and create a concept web about the atom. Work on your own. You have about 5 minutes. Jot down everything you can connect to atoms. Atoms Timeline of the Atom Democritus (460-370 B.C.) understood that if you cut a stone in two pieces, each piece contained the same material as the original stone. He also believed that you could do this an infinite number of times. He called these infinitesimally small pieces of matter atomos, meaning "indivisible.“ Rutherford’s gold foil experiment leads to atomic nucleus and in 1919 the introduction of the proton 1808 Dalton first proposed a theory on atoms Discovery of the electron by J.J. Thompson in the late 1890’s 1910 Lord Kelvin’s “plum pudding theory 1912 –Bohr model of the hydrogen atom Mid-1920’s – wave mechanical model 1932 – Rutherford and his coworker Chadwick identified the neutron Who started… John Dalton (1766 – 1844) was an English scientist who made his living as a teacher in Manchester. Dalton’s Atomic Theory (p.88) Elements are composed of atoms All atoms of a given element are identical Atoms of a given element are different from those of any other element Atoms of one element combine with atoms of other elements to form compounds Law of Constant Composition: all samples of a compound have the same proportion of the elements as in any other sample of that compound Atoms are indivisible in a chemical process. all atoms present at the beginning of a chemical process must also be present at the end of the process. atoms are not created or destroyed, they must be conserved. atoms of one element cannot be turned into atoms of another element Atomic Structure History Discovery of the Electron 1st atomic particle identified In 1897, J.J. Thomson used a cathode ray tube to deduce the presence of a negatively charged particle. Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. This creates a beam of negatively charged particles bent by an electric field. Conclusions from the Study of the Electron Cathode rays have identical properties regardless of the elemental gas used to produce them. Therefore, all elements must contain identically charged particles (electrons). Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass An electron is a tiny, negatively charged particle The next step…. What is the positive charge? Models of the atom William Thomson’s (Lord Kelvin’s) Atomic Model Lord Kelvin believed that the electrons were like plums embedded in a positively charged “pudding,” thus it was called the “plum pudding” model (easier to think of as “chocolate chips" in chocolate chip cookie dough.) Rutherford’s Gold Foil Experiment Rutherford’s Gold Foil Experiment Rutherford shot α (alpha) particles at a thin sheet of gold foil (think: bullet = alpha particles, target atoms = gold foil) α particles are positively charged gold atoms are about 50 larger than α particles. Particles were fired at a thin sheet of gold foil Particles hit on the detecting screen (film) were recorded (a) The results that the metal foil experiment would have yielded if the plum pudding model had been correct. (b) Actual results known as Rutherford’s model. Over 98% of the particles went straight through About 2% of the particles went through but were deflected by large angles About 0.01% of the particles bounced off the gold foil Most of the volume of the atom is empty space Rutherford’s Conclusion: A Nuclear Model The atom contains a tiny dense center called the nucleus The nucleus is essentially the entire mass of the atom (extremely dense) The nucleus is positively charged The electrons move around in the empty space of the atom surrounding the nucleus Finally, the neutron.. Discovered in 1932 by Chadwick based on the idea from Rutherford Has no charge Is located in the nucleus Mass a mass of 1 amu (actually, it’s slightly larger than a proton but for our work the mass is the same) The Modern Atom We know atoms are composed of three main atomic particles - protons, neutrons and electrons The nucleus contains protons and neutrons The radius of the atom is about 100,000 times larger than the radius of the nucleus Summary of Atomic Particles Particle Charge Mass # Location Electron -1 0 Electron cloud Proton +1 1 Nucleus 0 1 Nucleus Neutron Going beyond the electron, proton, and neutron Describing an Atom How many protons, neutron, and electrons does an atom have? Atomic Structure - protons The number of protons in an atom of a given element is the same as its atomic number (Z). (Z) is found on the Periodic Table, whole # for each element # of protons Atomic # (Z) 6 6 Phosphorus 15 15 Gold 79 79 Element Carbon Atomic Structure - neutrons Mass number = protons + neutrons; always a whole number. # of Neutrons = mass number - # of protons Atomic mass to mass number– decimal number in each element’s box on the periodic table. If you round the atomic mass of an element to the closest whole number you get the mass # for that element. Atomic Structure - Electrons # of Electrons = # of protons if the atom is neutral If the chemical symbol is written with a charge, representing an ion, the charge indicates the number of electrons that have been added or removed from the atom. If the ion has a positive charge (cation), subtract that charge from the # of protons to get the number of electrons. If the ion has a negative charge (anion), add that charge number to # of protons to get the number of electrons. # of Electrons = # protons – charge Charge = # protons - # electrons Representing atomic particles in atoms The number of each type of atomic particle (proton, neutron, electron) is determined by using symbols. There are several different ways to write an element: Atomic symbols Nuclear symbols Atomic Symbols include the element symbol and a charge if any. C – neutral carbon C+4 – carbon cation C-4 – carbon anion Nuclear Symbols Mass number (p+ + no) Charge (if any) 238 92 Atomic number (number of p+) U + Element symbol Element name Cl-1 –38 Mass number Fluorine-18 Element symbol with charge Mass number Atom Silver – 109 Pb-208 C-14 # protons # neutrons # electrons Isotopes atoms of an element with the same number of protons but different numbers of neutrons Isotope Protons Electrons Neutrons Hydrogen–1 (protium) 1 1 0 1 1 1 1 1 2 Hydrogen-2 (deuterium) Hydrogen-3 (tritium) Nucleus Two isotopes of sodium. Isotopes Examples 3517Cl 17Cl 37 H-1 H-2 H-3 Copper – 63 Copper – 65 Isotopes and Atomic Mass AMU When we think about the mass of an atom, we use atomic mass units (amu). A proton is 1 amu A neutron is 1 amu Add up protons and neutrons to get the mass number (not atomic mass) Why? Most elements in nature have isotopes All these isotopes contribute to the average atomic mass (listed on the table) Determining Average Atomic Mass The average atomic mass seen on the periodic table is a combination of all an element’s isotopes and their abundance. To determine the average atomic mass for an element, you must 1. Multiply the percentage (percent abundance) of each isotope of the element by its mass number. 2. Add the products of the multiplications together. 3. Divide by 100. 4. Your answer should be very close to the atomic mass of the element for that element Average Atomic Mass Examples Find the average atomic mass of each of the following elements from their percentages and mass numbers. 69.17% 63Cu and 30.83% 65Cu 5.85% Fe-54, 91.75% Fe-56, 2.12% Fe-57 and 0.28% Fe-58 Nuclear Reactions What you just did was write nuclear reactions. Typical reactions are decay reactions and capture reactions. What did you notice about the products of the reactions? The products of the reactions are isotopes of the element. Nuclear reactions produce different particles that are not elements. You need these particles to balance out the protons and neutrons in the nuclei. In a nuclear reaction, the atomic number (Z) and the mass number (A) are conserved. Radioactive decay Radioactive decay is a natural process. A nuclei may spontaneously kick out a particle, forming a new element. There are four common particles: Only a handful (~200) of the known isotope nuclei (2000) do not decay The alpha particle - α (gold foil) The beta particle – β The gamma particle – γ The positron particle In many cases, the process does not stop at one step, but rather a combination of steps. This is known as a decay series. Capture Reactions Nuclear Transformations Nuclear transformation is the changing of one element to another (modern alchemy!!!) using larger atoms Rutherford observed 1st transformation in 1919 Marie Curie and her husband another transformation (14 years later) Electron capture is the “natural” nuclear transformation Man-made elements are made by bombarding two nuclei together The nuclear particles Radioactivity Review Radioactivity is expressed as either a decay process or a capture process Decay processes can be connected as a chain Nuclear transformations use larger atoms to create different elements than what you started with Review of nuclear symbols Mass number is in upper left of symbol Atomic mass is in lower left of symbol 238 92 U Nuclear particles are written in the nuclear format The alpha particle (α) The alpha particle is actually a helium nucleus. It is the weakest of the decay particles. A alpha particle has a mass number of 4 and an atomic number of 2. If an α particle is added to a nuclei, the mass number will increase by 4 and the atomic number by 2. If an α particle is released from a nuclei, the mass number will decrease by 4 and the atomic number by 2. For example: The Beta particle (β) The beta particle is an electron. It has no mass. It does have a charge Examples include: Sometimes a nucleus will grab an electron that is close. This is called electron capture. The Gamma Particle (γ) This is also know as the gamma ray. This is a high energy photon of light. Picked up by specific detectors. It is the strongest (most dangerous) of the decay particles. Example: The Positron The positron is similar to a beta particle, but has a positive charge. Example: Neutron Emission Neutron emission or decay does not change the element, only the mass Example: Balancing Nuclear Equations Mass # and the atomic # totals must be the same for reactants and the products. 3919K 3517Cl + ___ 20682Pb 0-1e + ___ Writing Balanced Nuclear Equations Alpha decay of Cu-68 Gamma emission of Thorium-235 Positron emission of P-18 Astatine-210 releasing 3 neutrons Electron capture with Ti-45 Nuclear Chemistry and half-life Balancing Nuclear Equations Half-life Half-Life and Nuclear Stability Radioactive isotopes or nuclides all decay because they are unstable, some just breakdown much faster than others Geiger counter or scintillation counters are used to detect particles. Half-life – amount of time for half of the original sample to decay For two samples of the same isotope, regardless of the sample size, after one half-life, only half of the original amount of sample remains. Sample Half-lives Isotopes Carbon – 14 Sodium – 24 Bismuth – 212 Polonium – 215 Thorium – 230 Thorium – 234 Uranium – 235 Uranium – 238 Half-Live 5730 years 15 hours 60.5 seconds 0.0018 seconds 75400 years 24.1 days 7.0 x 108 years 4.46 x 109 years Working with half-life A material has t1/2 = 10 minutes. If you begin with 16g, how long will it take to decay to 2 g? Begin with 16 g, 1 half life gets you to 8 g. 2 half lives get you to 4 g. 3 half lives get you to 2 g. So, 3 x 10 minutes = 30 minutes. A material has t1/2 = 150 years. If you begin with 100 g, how long will it take to decay to 3.125 g? A material has t1/2 = 15 minutes. How much material is left after 75 minutes if you begin with 100 g? Calculate the number of half-lives used = 75/15 = 5 Run through 5 half lives: After 1 – 50g After 2 – 25 g After 3 – 12.5 g After 4 – 6.25 g After 5 – 3.125 g A material has t1/2 = 6.2 years. How much material is left after 24.8 years if you begin with 14 g? What is the half-life of a material that decays from 16 g to 2 g over 20 minutes? Determine the number of half lives: 16 → 8 8→4 4→2 3 half lives spent Divide the time by the number of half-lives: 20/3 = 6.67 minutes What is the half-life of a material that decays from 125 g to 3.9 g over 100 years? Uses of nuclear chemistry Uses of Nuclear Chemistry Medicine X-rays and MRIs Chemical tracers Energy Destruction Fission versus Fusion Fusion – combining two smaller nuclei into one heavier, more stable nucleus. 3 He + 1 H 4 He + 0 e 2 1 2 1 Fission – splitting a large unstable nucleus into two nuclei with smaller mass numbers. 209 Po 125 Te + 84 Ge 84 52 32 Sample fission reaction Sample fusion reaction