R The Periodic Table
advertisement
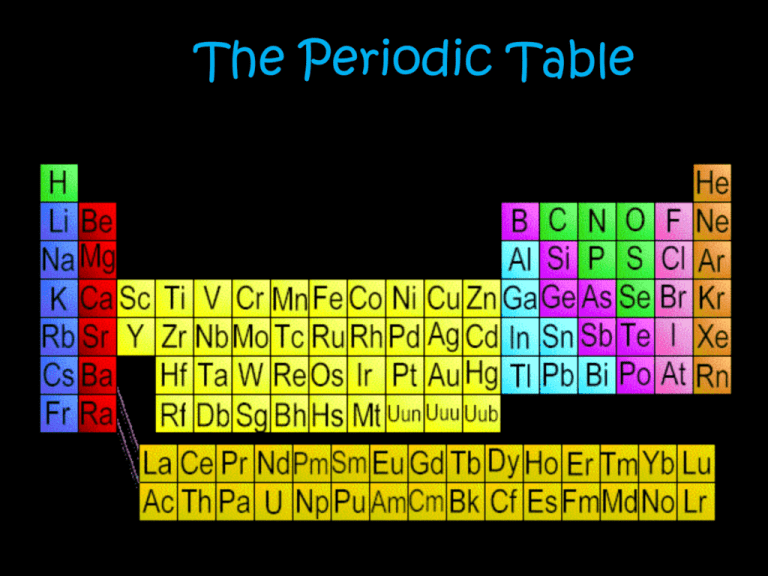
R The Periodic Table D’Mitri Mendeleev • 1860’s • First to publish • Placed similar elements in same vertical column • Left open spaces for elements he predicted would someday be discovered Describing the Periodic Table • The periodic table is a chemistry reference • It arranges all the known elements in an informative grid • Elements are arranged left to right and top to bottom in order of increasing atomic number • This order usually coincides with increasing atomic mass Describing the Periodic Table • The Table's pattern and arrangement shows the arrangement of electrons in the atom. • Elements have different atomic numbers - the number of protons or electrons increases up the table as electrons fill the shells. • Elements have different atomic masses - the number of protons plus neutrons increases up the table. Families on the Periodic Table • Elements on the periodic table can be grouped into families bases on their chemical properties. • Each family has a specific name to differentiate it from the other families in the periodic table. • Elements in each family react differently with other elements. Columns • The vertical (up and down) columns of the periodic table (there are 18) are called groups or families. • Elements in the same group or family have similar characteristics or properties. Rows • The horizontal rows of the periodic table are called periods. • Elements in a period are not alike in properties. • The first element in a period is usually an active solid, and the last element in a period is always an inactive gas. Rows • Atomic size (number of protons) increases from left to right across a period. • Atomic mass (number of protons & Neutrons) increases from left to right across a period. • The period number of an element signifies the highest energy level an electron in that element occupies. Rows • Electrons are added one at a time moving from left to right across a period. • The electrons on the outermost shell have increasingly strong nuclear attraction, so the electrons become closer to the nucleus. Describing the Periodic Table Interpreting the Periodic Table Interpreting the Periodic Table Interpreting the Periodic Table • The number of protons in an atom tells what element it is. • The number of protons in an atom is referred to as the atomic number of that element. Interpreting the Periodic Table Atomic Symbol: • The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). • These symbols are used every where in the world • Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element. Interpreting the Periodic Table Atomic Mass: • The atomic mass is the average mass of an element in atomic mass units ("amu"). • Though individual atoms always have a whole number of amu’s, the atomic mass on the periodic table is shown as a decimal number because it is an average of all the isotopes of an element. Interpreting the Periodic Table Mass Number: • The sum of the protons and neutrons that make up that nucleus. • The mass number is different for each isotope of an element. Groups and Periods Alkali Metals • Very reactive metals that do not occur freely in nature • Malleable, ductile, good conductors of heat and electricity, solids at room temperature • Have low densities and low melting points • Softer than most other metals • Can explode if they are exposed to water • Used to produce chemicals, metals, soap, glass, ceramics, and petroleum products Groups and Periods Alkaline Earth Metals • Metals • Very Reactive (give up 2 electrons during reactions) • Never found free in nature Groups and Periods Transition Metals • ductile and malleable, and conduct electricity and heat • iron, cobalt, and nickel, are the only elements known to produce a magnetic field • Used in jewelry, industry (copper, steel, iron), technology, light bulbs, and in the food we eat • Less reactive than most metals Groups and Periods Other Metals • are ductile and malleable • are solid, have a relatively high density, and are opaque Groups and Periods Metalloids •Have properties of both metals and nonmetals •Appearance will vary •Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators. • Conduct better than nonmetals but not as well as metals •NOTE: any element above 92 is too unstable to occur in nature and must be done in a lab, scientist were able to predict existence of elements (neon and germanium before they were even discovered Groups and Periods Nonmetals Metals •not able to conduct electricity or heat very well •exist in two of the three states of matter at room temperature: gases (such as oxygen) and solids (such as carbon). •very brittle, and cannot be rolled into wires or pounded into sheets •have no metallic luster, and do not reflect light. Halogens Groups and Periods • halogen" means "salt-former" and compounds containing halogens are called "salts" • Very reactive nonmetals that form salts when combined with many metals • Very reactive; often bond with elements from group one. • Used to kill harmful micro-organisms in hospitals, to purify drinking water, and prevent growth of algae in swimming pools. •exists in all three states •Solid- Iodine, Astatine •Liquid- Bromine •Gas- Fluorine, Chlorine Groups and Periods Noble Gases • Exist as gases, non-metals • 8 electrons on outer shell= full • Not reactive with other elements. Groups and Periods Rare Earth Metals • The Rare Earth Metals are typically silver, silverywhite, or gray metals. • Good conductors of electricity • 2 Subgroups – Lanthanide Series: many forms, some with magnetic properties – Actinide Series • All are radioactive






