States of Matter PowerPoint
advertisement

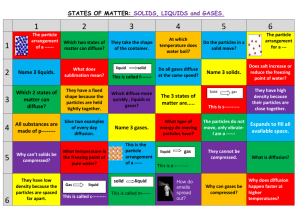
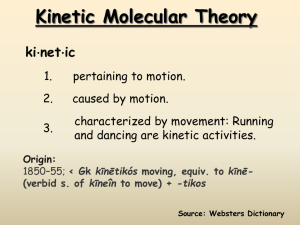
States of Matter A Matter of Kinetic Energy Types of States of Matter • • • • • Solid Liquid Gas Plasma BEC, or Bose-Einstein Condensate – Zero State of Matter – Most Dense Changes of State Kinetic Energy (kelvins & paschals) chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_ of_Matter/Supercritical_Fluids • Supercritical fluids are useful in science today – extraction of floral fragrance – the process of creating decaffeinated coffee – food science and functional food ingredients – pharmaceuticals, cosmetics, polymers, powders, bio- and functional materials – nano-systems, natural products, biotechnology, fossil & biofuels, microelectronics & environment (Bottini 133). www.engineeringtoolbox.com/vapor-steam-d_609 • Superheated Vapor • When the temperature is higher than the boiling point @ a given pressure. • Vapor cannot exist in contact with the fluid, nor contain fluid particles. • Increase in pressure or decrease in temperature will not, within limits, condensate out liquid particles in the vapor. • Highly superheated vapors are gases that approximately follow the general gas law. Critical Temp & Pressure • Critical Temperature – The temperature at which only gas exists, regardless of its pressure • Critical Pressure – The lowest pressure at which liquids exist at critical temperature • Critical Point – The intersection of critical temperature & pressure CO2 Phase Diagram Kinetic-Molecular Theory of Gases • Ideal gas = hypothetical gas perfectly aligns with all kinetic-molecular theory assumptions • Five Assumptions – Distance between molecules dwarfs actual size – All collisions are perfectly elastic – Particles are in continuous, rapid, random motion – Particles have NO attraction to each other – Temperature = average kinetic energy of particles Nature of Gases • Ideal vs. Real – Real approaches ideal @ low pressure/ high temp • • • • • Expansion – molecules fill entire space Fluidity – no intermolecular attractions Density - ~ 10-3 of liquid or solid state Compressibility – 100X more than liquids Diffusion & Effusion – Diffusion: Spontaneous mixing via random motion – Effusion: Passing through tiny opening Properties of Liquids • • • • LEAST common state of matter in universe Fluids (as are gases) Lower kinetic energy than gases Interactive forces keep molecules connected – Dipole-dipole forces • Equal but opposite charges separated by short distance – London dispersion forces • Spontaneous creation of dipoles (polar & nonpolar) – Hydrogen bonding (electronegativity) Properties of Liquids, continued • • • • • Density: 100x > gases; 10% < solids Compressibility: @ 103 atm., volume ~ 4% Diffusion: present, but slower than in gases Surface tension: high intermolecular attraction Capillary action: attraction between surfaces of liquid and a solid • Vaporization: evaporation & boiling gas Nature of Solids • Interparticle attractions stronger than others • Two types of solids – Crystalline (orderly arrangement) – Amorphous (random arrangement) • supercooled liquids: have liquid properties even if look solid (like glass and plastics) • • • • Shape & Volume: Definite Melting Point: Definite Density & Incompressibility: High Diffusion: Low rate (10-6 less than others) Crystalline Solids • Ionic • Alkali & alkaline earth with halogens & Group 16 • Hard, brittle, high melting points, good insulators • Covalent network • Cx (diamonds), (SiO2)x quartz, (SiC)x • Very hard and brittle, high MP, semi- or nonconductors • Covalent molecular (nonpolar & polar) • H2, CH4, C6H6: only weak London dispersion forces • H2O & NH3,: stronger forces but weaker than covalent • Soft, low MP, low BP, good insulators