QUIZ_GRID_States_of_matter
advertisement
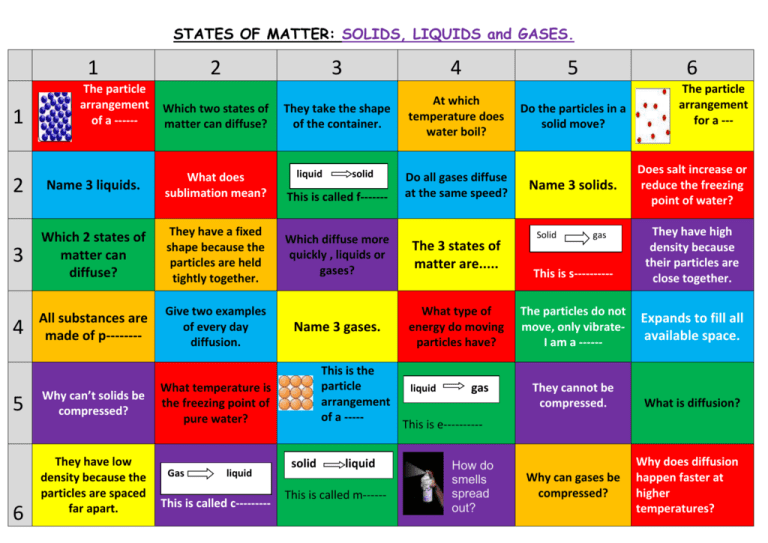
STATES OF MATTER: SOLIDS, LIQUIDS and GASES. 1 1 The particle arrangement of a ------ 2 Which two states of matter can diffuse? 2 Name 3 liquids. What does sublimation mean? 3 Which 2 states of matter can diffuse? They have a fixed shape because the particles are held tightly together. All substances are made of p-------- Give two examples of every day diffusion. 4 5 6 Why can’t solids be compressed? They have low density because the particles are spaced far apart. 3 4 They take the shape of the container. At which temperature does water boil? liquid This is called f------- liquid This is called c--------- Do all gases diffuse at the same speed? Which diffuse more quickly , liquids or gases? The 3 states of matter are..... Name 3 gases. What type of energy do moving particles have? This is the particle liquid gas his is called arrangement of a ----This is e---------- What temperature is the freezing point of pure water? Gas solid solid liquid This is called m------ How do smells spread out? 5 6 Do the particles in a solid move? Name 3 solids. The particle arrangement for a --- Does salt increase or reduce the freezing point of water? This is s---------- They have high density because their particles are close together. The particles do not move, only vibrateI am a ------ Expands to fill all available space. They cannot be compressed. What is diffusion? Solid gas Why can gases be compressed? Why does diffusion happen faster at higher temperatures?