Chapter 9: Molecular geometry and bonds
advertisement
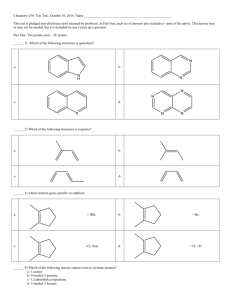
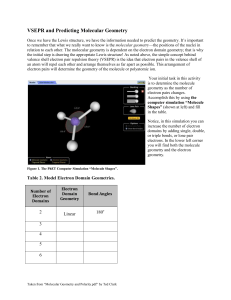
CHAPTER 9: MOLECULAR GEOMETRY AND BONDS JANUARY 28TH, 2013 Do Now: Calculate the ∆H: C2H4 + HCN CH3CH2CN MOLECULE SHAPE How do we determine the shape of a molecule? How are bonds related to the location of electrons? Draw CO2, how many electron domains are located on the central carbon? VSEPR THEORY States: “Best arrangement of a given number of electron domains is the one that minimizes the repulsions among them” Predict the difference between electron-domain geometry and molecular geometry. How do we use VSEPR Theory: Draw lewis structure of molecule or ion and count number of electron domains around central atom. Determine electron-domain geometry Using table determine (eventually *memorize*) molecular geometry PRACTICE Predict the molecular geometry of: O3 SnCl3 Compare and contrast: H2O CH4 NH3 Explain the ideal bond angles. WORK IT OUT How are all the bond angles related to one another? With a partner or small group, discuss why it is possible that all these angles are so large, similar. In fancy terms: Non-bonding electrons give off greater repulsive forces therefore compressed bond angles. ** NOTE: double bonded atoms tend to exert a greater repulsive force as well. PRACTICE: Determine the following molecular geometries: SF4 IF5 BrF3 ICl4 LARGER THAN LIFE… Large molecules need to be broken down into their smaller components: Draw CH3COOH (use VSEPR for each central atom) POLARITY Why is polarity important? How do we determine polarity? Bonds: Molecules: Predict the polarity of the following: BrCl SO2 SF6 EXPLANATIONS How do we explain bonding? How is energy related to distance of covalent bonds? Why is there a sharp increase with shorter distance? All in all: bond length is distance at which the attractive forces between unlike charges are balanced by repulsive forces within the molecule