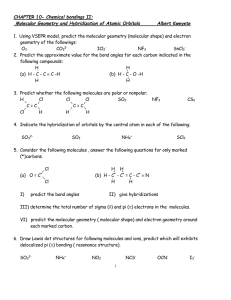
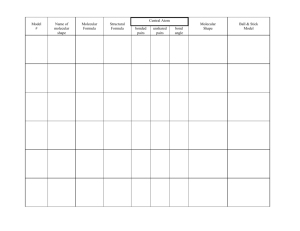
Homework #6: Molecular geometry
advertisement
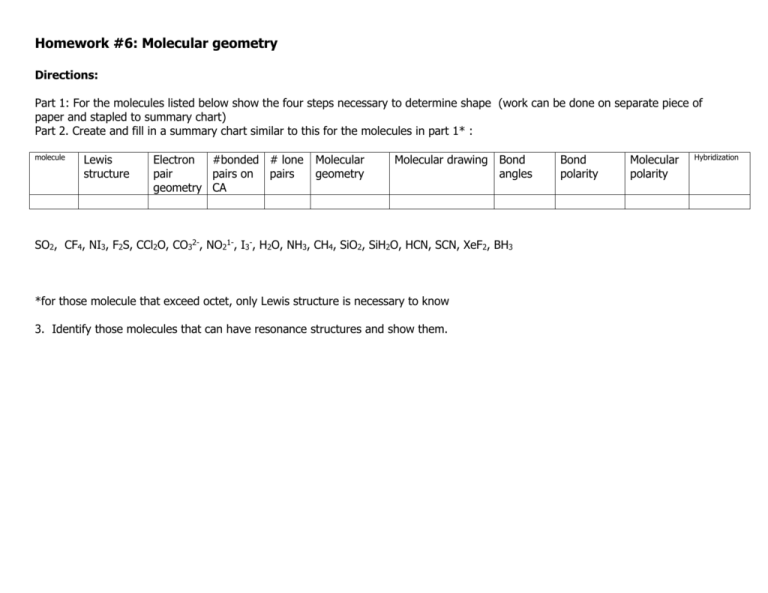
Homework #6: Molecular geometry Directions: Part 1: For the molecules listed below show the four steps necessary to determine shape (work can be done on separate piece of paper and stapled to summary chart) Part 2. Create and fill in a summary chart similar to this for the molecules in part 1* : molecule Lewis structure Electron #bonded # lone pair pairs on pairs geometry CA Molecular geometry Molecular drawing Bond angles SO2, CF4, NI3, F2S, CCl2O, CO32-, NO21-, I3-, H2O, NH3, CH4, SiO2, SiH2O, HCN, SCN, XeF2, BH3 *for those molecule that exceed octet, only Lewis structure is necessary to know 3. Identify those molecules that can have resonance structures and show them. Bond polarity Molecular polarity Hybridization