Chapter 7
advertisement

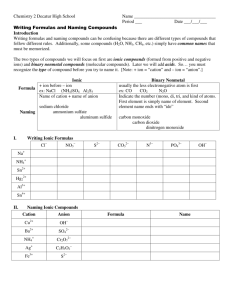
Chapter 7 REALLY Important!!! 7.1 – Ionic Compounds: Ions for s and p block elements: Group 1 H Li Na K Rb Cs Fr Group 2 Be Mg Ca Sr Ba Ra Group13 B Al Ga In Tl Group 14 C Si Ge Group 15 N P Group 16 O S Group 17 F Cl Br I At ________ Valence e- ________ Valence e- ________ Valence e- ________ Valence e- ________ Valence e- ________ Valence e- ________ Valence e- Dot Diag: Dot Diag: Dot Diag: Dot Diag: Dot Diag: Dot Diag: Dot Diag: Wants to _________ Wants to _________ Wants to _________ Wants to _________ Wants to _________ Wants to _________ Wants to _________ All ions are ________ All ions are ________ All ions are ________ All ions are ________ All ions are ________ All ions are ________ All ions are ________ Names: Names: Names: Names: Names: Names: Sn Pb As Sb Bi Se Te Po Dot Diag: Dot Diag: Dot Diag: Can lose 2 Can lose 3 Can lose 4 Can lose 4 Can lose 5 Can lose 6 Names: 7.1 – Ionic Compounds: Ions – d Block *Intro Classes can put Roman Numerals on ALL d-block elements *Honors Class must know which elements NEED Roman Numerals cobalt (II) = Co+2 cobalt (III) = Co+3 Cu+1 copper (I) = copper (II) = Cu+2 chromium (II) = Cr+2 chromium (III) = Cr+3 chromium (VI) = Cr+6 iron (II) = Fe+2 iron (III) = Fe+3 Silver (I) = Ag+1 platinum (II) = Pt+2 platinum (IV) = Pt+4 mercury (I) = *Hg2+2 (Academic: Hg+1) mercury (II) = Hg+2 Zinc (II) = Zn+2 cadmium (II) = Cd+2 manganese (II) = Mn+2 manganese (IV) = Mn+4 nickel (II) = Ni+2 nickel (III) = Ni+3 gold (III) = Au+3 7.1 – Polyatomic Ions The Tough Stuff! THE 8 -ATES: • Carbonate • Nitrate • Sulfate • Chlorate • Chromate • Bromate • Phosphate • Iodate CO3-2 NO3-1 SO4-2 ClO3-1 CrO4-2 BrO3-1 PO4-3 IO3-1 Rules with –ates: 1 more oxygen than –ate = per … ate 1 less oxygen than –ate = …ite 2 less oxygens than –ate = hypo … ite 7.1 – Other Polyatomic Ions Ammonium Hydronium Peroxide Hydroxide Cyanide NH4+1 H3O+1 O2-2 OH-1 CN-1 Ions Test Ends Here……… 7.1 – Ionic Compounds – Names and Formulas Naming Binary Ionic Compounds: A metal + a nonmetal = IONIC Name = cation name anion name (w/-ide ending) -Use Roman Numerals if needed Examples: 7.1 – Ionic Compounds – Names and Formulas Writing Binary Ionic Formulas: Remember Chapter 6? New way: Use the swap technique – Number on charge tells you how many of the OTHER element Examples: More Examples 7.1 – Ionic Compounds – Names and Formulas Naming Ionic Compounds w/more than 2 elements: Name = cation name anion name (at least one will be a polyatomic ion) Examples: 7.1 – Ionic Compounds – Names and Formulas Writing Ionic Formulas for Ionic Compounds with More Than 2 Elements: Use the swap technique Examples: Ionic Compound Review Formula of: Calcium bromide Copper (II) sulfide Nickel (II) phosphate Strontium hydroxide Barium nitrite Name of: CaO MgCl2 CrO3 Fe3(PO4)2 Al2(CO2)3 7.1 – Molecular Compounds 2 Nonmetals Bonded USE PREFIXES! Mono= Di= Tri= Tetra= Penta= Hexa= Hepta= Octa= Nona= Deca= 7.1 - Molecular Compounds Name = prefix first element prefix second element-ide Prefix is the quantity of that element Mono is not needed in front of the FIRST element only Examples: 7.1 – Review Practice Problems -Ions -Naming Ionic Compounds -Writing Ionic Compound Formulas -Naming Molecular Compounds -Writing Molecular Compound Formulas 7.2 – Oxidation Numbers -Show distribution of electrons -Negative means “stronger element / taking electrons” and positive means “weaker element / losing electrons” Rules: 1. Uncombined element has oxidation number = 0 2. Monatomic ion has oxidation number = charge 3. If in a compound: A. Start with the element on the right. It has oxidation number = charge if it was a negative ion. B. If more than 2 atoms, go next to the element on the left, it has oxidation number = charge if it was a positive ion. C. Calculate the remaining element (It may not match what you memorized as the ion charge) 4. Sum of all oxidation numbers in a neutral compound = 0 5. Sum of all oxidation numbers in a polyatomic ion = charge on ion 7.2 – Oxidation Numbers Practice Problems: 7.3 – Molar Mass Molar Mass = Sum of Average Atomic Masses for all elements in a compound. Unit = g/mol Examples: End after first example Using Molar Mass Can be used as a conversion factor O2 = 32 grams / 1 mole OR 1 mole / 32 grams Examples: Percent Composition Percent by mass of an element in a compound (Mass element / molar mass) x 100 = % composition Examples: EXPERIMENTALLY Determining Formulas Empirical Formula = The simplest (most reduced) formula Example: The empirical formula of glucose (C6H12O6) is…. Steps: 1. Find moles of each element 2. Write formula with subscripts 3. Divide all moles by the smallest number of moles 4. If .5s multiply all by 2 Empirical Calculations Examples: EXPERIMENTALLY Calculating Molecular Formulas This is the NON-Reduced formula (ex – glucose = C6H12O6) Need to have the Empirical Formula and the molecule’s Molar Mass Examples: -If the empirical formula is BH3, and the molar mass of the compound is 27.7 g/mol, what is the Molecular Formula?