Writing Formulas for Ionic Compounds
advertisement

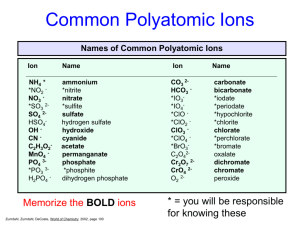
Writing Formulas for Ionic Compounds General Rules 1. Write each ion symbol with charge: simple ions from front of periodic table; complex on back. 2. Find lowest common denominator for the charges of the 2 ions. For each ion, multiply the ion charge by a number that will give the common denominator. The following must apply: Total positive charge = total negative charge. Write in the correct subscripts after each ion symbol – this will be the number of each ion that are needed to have charges equal. Use lowest whole number ratios. Cations come first in the formula, anions come second. Naming Ionic Compounds General Rules: Name each ion o Cations are named first in the formula, anions are named second. Types of Ions and Ionic Compounds There are 4 different types of ions that may be found in Ionic compounds. A. Monatomic Ions (Simple Ions) Single atoms that have lost or gained one or more electrons Form Binary Ionic Compounds (2 simple ions) Eg. Na+ Cl- B. Polyatomic Ions (Complex Ions) Cations or anions composed of a group of atoms with a net positive or negative charge Eg. NH4+ NO2NO3CO32- Ammonium ion Nitrite ion Nitrate ion Carbonate ion C. Multivalent Ions certain transition metals can form more than one type of ion, each with a different charge Eg. Fe3+ Fe2+ The more commonly occurring ion is listed on top, thus Fe3+ is more common than Fe2+ D. Hydrated Ionic Compounds Water molecules are loosely held within the ionic compound Eg. ZnCl2 • 6H2O CuSO4 • 5H2O Monoatomic Ionic Formulas Examples silver chloride aluminum oxide barium fluoride lithium sulfide magnesium nitride Monoatomic Ionic Compound Names NOTE: When naming monotomic ionic compounds, the cation retains metal name, and the anion name ends in "ide" Examples Ca3P2 AlCl3 BeI2 Al2S3 BaBr2 Writing Formulas for Polyatomic Ionic Compounds The same general rules apply, however: If more than one complex ion is present, place brackets around it, then give its subscript. Examples: ammonium nitrate NH4NO3 potassium carbonate K2CO3 sodium thiosulfate Na2S2O4 lithium hydrogen carbonate LiHCO3 Naming Polyatomic Ionic Compounds The same general rules apply, where cations are named first in the formula, anions are named second, however: Note: Names of polyatomic ions are on the back of the periodic table at the top Examples: Na3BO3 sodium borate (NH4)2CO3 ammonium carbonate Na2Cr2O7 sodium dichromate KCrO4 potassium chromate Writing Formulas for Multivalent Ionic Compounds The same general rules apply, however: The Roman numerals indicate the charge on the multivalent ion. Examples: Roman Numerals iron(II)sulfide lead(IV)oxide gold (IV) phosphide uranium (VI) oxide I = one II = two III = three IV= four V = five VI = six VII = seven Naming Multivalent Ionic Compounds The same general rules apply, however: You must use Roman numerals in the name to indicate charge of the particular multivalent ion Examples: FeO V3N5 AuCl3 CrBr3 Writing Formulas for Hydrated Ionic Compounds The same general rules apply, however: Numbers and the formula “ · ___H2O” will be used to indicate the number of water molecules are given in the name of the hydrated ionic compound. Numerical Prefixes 1 = mono 2 = di 3 = tri 6 = hexa 7 = hepta 8 = octa 4 = tetra 5 = penta 9 = nona 10 = deca Examples: zinc chloride hexahydrate sodium chlorite heptahydrate lithium nitrite pentahydrate nickel (II) bromide decahydrate Naming Hydrated Ionic Compounds The same general rules apply, however: Numerical prefixes and the word “______hydrate” will be used to indicate the number of water molecules given in the formula. Examples: Ba(OH)2 · 8H2O Na2Cr2O7 . 5 H2O AgNO3 . 4H2O FeSO4 . 5H2O