Chapter Twelve PPT
advertisement

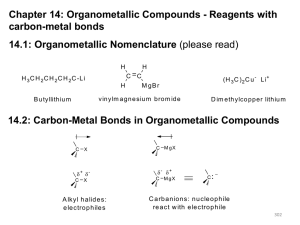
Alcohols, Carbonyls and REDOX • The Carbonyl Group (Section 12.1) • Oxidation/Reduction Reactions: Review (Section 12.2) • Reduction of Carbonyls to Alcohols (Section 12.3) • Oxidation of Alcohols (Section 12.4) • Organometallic Compounds (Section 12.5) • Organolithium and Magnesium Compounds (Section 12.6) • Reactions of Organolithium/Magnesium Species (Section 12.7) • Alcohols from Grignard Reactions (Section 12.8) • Lithium Dialkylcuprates (Section 12.9) The Carbonyl Functional Group O O R Carbonyl O O H R Aldehyde R' Ketone R O OH Carboxylic Acid R OR' Carboxylate Ester O 120o R R Planar, sp2 Hybridized Carbon • Carbonyl Features 1 s and 1 p Bond • Carbonyl Group Quite Polarized (Cd+, Od-) Resonance Structure for Carbonyl Reflecting Bond Polarization?? General Reactions of Carbonyls Nucleophilic Addition to Carbonyl Groups: d Nu Nucleophile Nu d O O Carbonyl Addition Product: TETRAHEDRAL Oxidation of Alcohols/Reduction of Carbonyls: O More Hydrogen Content Oxidation R OH Primary Alcohol Reduction R H Aldehyde Less Hydrogen Content Oxidation/Reduction Reactions • Commonly Termed ‘REDOX’ Reactions • From General Chemistry, we Will Recall Oxidation: Loss of Electrons Reduction: Gain of Electrons • Organic Chemists will Typically use Different Definitions Reduction: Increase Hydrogen Content (Decrease Oxygen) Oxidation: Decrease Hydrogen Content (Increase Oxygen) • Oxidizing/Reducing Agents: Usually Inorganic Compounds (M+) • We will also Recall that in REDOX Reactions: Oxidizing Agents get Reduced Reducing Agents get Oxidized Oxidation States of Carbon: Organics H CH3 H CH3 H C H H3C C H H -4 CH3 C H H CH3 H -3 C CH3 H -2 -1 CH3 H3C C CH3 H3 C 0 O Br O O H 3C C C C CH3 H3 C 1 H3C C CH3 2 H3C OH 3 O 4 • +1 For More Electronegative, -1 For Less, 0 For Bonded Carbon Alcohol Synthesis: Carbonyl Reduction O [H] Reduction R OMe O R [H] Reduction OH ROH 1o Alcohol ROH 1o Alcohol O [H] Reduction R H R [H] Reduction Me Ketones Reduced to 2° Alcohols ROH 1o Alcohol OH O Carboxylic Acids, Esters, Aldehydes Reduced to 1° Alcohols R Me 2o Alcohol Several Hydrogen Sources Are Used In Organic Reactions: We’ve Already Seen NaBH4 Reducing Agents: 1° and 2° Alcohols • Sodium Borohydride: NaBH4 • Lithium Aluminum Hydride: LiAlH4 (LAH) • H2/Transition Metal Catalyst (z.b. CuO•CuCr2O4) • NaBH4 and LiAlH4 are Hydride Transfer Agents • Hydride (H¯) Acts as a Nucleophile • Carbonyls Have Varying Degrees of Ease of Reduction: O O O > R O Hardest O > R OR' > R R' R H Easiest Selection of a Reducing Agent • Choice of Reducing Agent Impacts Reaction Products • For Ketones/Aldehydes Either Reductant Suffices Carboxylate Ester Ketone Aldehyde 1° Alcohol 1° Alcohol 2° Alcohol 1° Alcohol NaBH4 No Reaction No Reaction 2° Alcohol 1° Alcohol LiAlH4 • Carboxylates/Esters Only Reduced by LiAlH4 • For Compounds w/ Multiple Carbonyl F.G.s; Select Based on Which Group(s) Need to be Reduced NaBH4/LiAlH4 Reduction Examples O OH OH 1. LAH/Et2O NaBH4 H2O 2. H2O/H2SO4 OH NO REACTION 1. LAH/Et2O NaBH4 H2O 2. H2O/H2SO4 O OH O OH 1. LAH/Et2O NaBH4 H2O O OH OH 2. H2O/H2SO4 O OH OH Oxidizing Agents in Organic Chemistry CrO3/H2SO4 N H CrO3Cl H2CrO4 Pyridinium chlorochromate (PCC) Chromic Acid (Jones Reagent) • PCC Generally a Mild Oxidant (1° Alcohol Aldehyde) • Jones Reagent Harsher Oxidant (1° Alcohol Carboxylic Acid) • Alcohol Often Dissolved in Acetone While Jones Reagent Added • Choose Oxidant Based on Desired Carbonyl Functional Group General Oxidizing Agent Selection • Just as in Reductions, Oxidation Products Depend on Reagent • Generally Don’t Oxidize 3° Alcohols (No Texas Carbons) MeOH 1° Alcohol 2° Alcohol 3° Alcohol PCC H2C=O Aldehyde Ketone No Reaction Cr6+ H2SO4 HCO2H Carboxylic Acid Ketone No Reaction • PCC Good For Aldehydes From Primary Alchols • Cr6+/H2SO4 Reagents, KMNO4 Primary Carboxylic Acids • Use What You Like For Most Ketones Oxidation of 1°, 2° Alcohols O OH PCC H CH2Cl2, 25 oC O OH H2CrO4 acetone, 35 oC OH OH KMnO4, H2O NaOH, Heat O Oxidation Mechanisms: Chromate Esters H Protonation, Followed by Loss of Water (Combined Here) H O O H O O HO Cr Cr O O O O H H O H H H2O O O + Cr OH O H O + H3O O Cr OH Chromate Ester O Organometallic Compounds • Organic Compounds Containing Carbon—Metal Bonds • Bonds Range From Ionic to Primarily Covalent • Ionic C—M Bonds: C—Na C—K • Primarily Covalent C—M Bonds: C—Pb C—Sn C—Hg • Inetermediate C—M Bonds Include C—Mg and C—Li • Reactivity Increases with Ionic Character of C—M Bond Organolithium Reagents Common Solvents for Organolithium Reagents: O Diethyl Ether O Tetrahydrofuran Preparation of Organolithium Reagents: Br 2Li, -10 oC Et2O Li + LiBr Butyllithium (Alkyl Lithium Reagent) • Reactive, Carbanion-Like Species (React Slowly w/ Ethers) • Halide Reactivity: RI > RBr > RCl (F Not Often Used) Grignard Reagents Preparation of Grignard Reagents: Br Mg Et2O MgBr Butylmagnesium Bromide (Grignard Reagent) MgBr Br Mg Et2O Phenylmagnesium Bromide (Grignard Reagent) • Reactivity of Halides Same as for Organolithium Reagents • Generally Exist as Complexes, We’ll Use RMgX for Simplicity Organometallic Reactions: Notes • Can Act as Nucleophiles Towards Polarized Carbonyl Groups • Very Strong Lewis Bases (React with Acidic Protons) • Basicity Necessitates Dry Conditions (Avoid Reaction w/ H2O) • Reason For Basicity: Carbanion-Like Behavior (pKa??) • Strong Enough Bases to Deprotonate Terminal Alkynes (pKa??) • With No Acidic Protons, Can Do Nucleophilic Substitution Let’s Look at Some Representative Grignard Reactions Grignard Reactions: Epoxides MgBr MgBr O 1. Et2O 2. H3O+ OH O 1. Et2O 2. H3O+ OH + + • Grignard Reagents Nucleophilically Open Epoxides • Generally Attack Less Substituted Carbon (Steric Hindrance) • View This as Carbanion Attacking in SN2 Reaction (O L.G.) Grignard Reactions w/ Carbonyls • Grignard Reagents React With a Variety of Carbonyls Formaldehyde 1° Alcohols Higher Aldeydes 2° Alcohols Ketones 3° Alcohols Ester 3° Alcohols • Attack of Grignard Generates Alkoxide; Protonate to get OH Let’s Look at Some Specific Grignard Reactions w/ Carbonyls Grignard Reactions: Carbonyls MgBr O OH Et2O + H MgBr H Me O OH Et2O + H3C H Me Me MgBr O Et2O + H3C CH3 OH Grignard Reactions: Esters Me MgBr OH O Et2O + H3C OCH3 • Grignard Reagents React Twice w/ Esters 3° Alcohols • Two Alkyl Groups of Alcohol Correspond to Grignard Reagent • Grignard Reactions Quite Useful in Wide Range of Alcohol Syntheses (w/ Varying Degrees of Substitution) Reactions of Organolithium Compounds • Organolithium Reagents React Similarly to Grignards • Also Strong Bases, Same Limitations Apply • More Reactive Species Than Grignard Reagents • Routine Syntheses: Prefer to use Grignard Reagents • Sodium Alkynides (Triple Bond Anions) React in Same Manner w/ Aldehydes and Ketones Now We’ll Look at One More Organometallic: Lithium Dialkylcuprates (A Coupling Reagent) Lithium Dialkylcuprates Me I (CH3)2CuLi Et2O CH3Br 2Li, Et2O 2 CH3Li CuI (CH3)2CuLi Me Br (CH3)2CuLi Et2O Quite Versatile C—C Bond Forming Reaction