Project Overview
advertisement

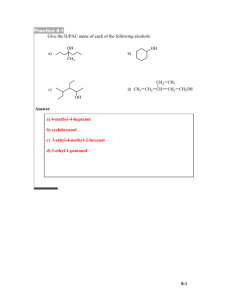
Chapter 12 Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Ch. 12 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 12 - 2 1. Structure of the Carbonyl Group Carbonyl compounds O R O H R Aldehyde R' Ketone O R O O O OH Carboxylic acid R OR' Ester R N R' Amide R" Ch. 12 - 3 Structure ~ 120o O o C ~ 120 ~ 120o ● Carbonyl carbon: sp2 hybridized ● Planar structure Ch. 12 - 4 Polarization and resonance structure O C O C Ch. 12 - 5 1A. Reactions of Carbonyl Compounds with Nucleophiles One of the most important reactions of carbonyl compounds is nucleophilic addition to the carbonyl group O Nu C O nucleophilic addition Nu C Ch. 12 - 6 Two important nucleophiles: ● Hydride ions (from NaBH4 and LiAlH4) ● Carbanions (from RLi and RMgX) Another important reactions: OH R H O oxidation H 1o alcohol reduction R C H aldehyde Ch. 12 - 7 2. Oxidation-Reduction Reactions in Organic Chemistry Reduction of an organic molecule usually corresponds to increasing its hydrogen content or decreasing its oxygen content oxygen content decreases O R carboxylic acid O O [H] OH reduction hydrogen content decreases R H R aldehyde OH [H] H reduction R H H Ch. 12 - 8 The opposite reaction of reduction is oxidation. Increasing the oxygen content of on organic molecule or decreasing its hydrogen content is oxidation OH [O] RCH3 lowest oxidation state [H] R H O [O] H [H] R O [O] H [H] R OH highest oxidation state Ch. 12 - 9 Ar Oxidation of an organic compound may be more broadly defined as a reaction that increases its content of any element more electronegative than carbon CH3 [O] [H] Ar CH2Cl [O] [H] Ar CHCl2 [O] Ar CCl3 [H] Ch. 12 - 10 2A. Oxidation States in Organic Chemistry Rules ● For each C–H (or C–M) bond -1 ● For each C–C bond 0 ● For each C–Z bond +1 (where M = electropositive element and is equivalent to H, e.g. Li, K, etc.; Z = electronegative heteroatom, e.g. OR, SR, PR2, halogen, etc.) Calculate the oxidation state of each carbon based on the number of bonds it is forming to atoms more (or less) electronegative than carbon Ch. 12 - 11 Examples H (1) H C H Bonds to C: 4 to H = (- 1) x 4 = - 4 H Total = - 4 Oxidation state of C = - 4 Ch. 12 - 12 Examples H (2) H C H Bonds to C: OH 3 to H = - 3 1 to O = +1 Total = - 2 Oxidation state of C = - 2 Ch. 12 - 13 Examples O (3) H C Bonds to C: H 2 to H = - 2 2 to O = +2 Total = 0 Oxidation state of C = 0 Ch. 12 - 14 Examples O (4) H C Bonds to C: OH 1 to H = - 1 3 to O = +3 Total = +2 Oxidation state of C = +2 Ch. 12 - 15 Overall order H H C H H < H oxidation - 4 state lowest oxidation state of carbon H C H -2 O OH < H C 0 O H < H O C OH +2 < C O +4 highest oxidation state of carbon Ch. 12 - 16 3. Alcohols by Reduction of Carbonyl Compounds H R [H] O OH R OR' H OH (1o alcohol) [H] O O R R O R H [H] [H] R' HO R H R' Ch. 12 - 17 3A. Lithium Aluminum Hydride LiAlH4 (LAH) ● Not only nucleophilic, but also very basic ● React violently with H2O or acidic protons (e.g. ROH) ● Usually reactions run in ethereal solvents (e.g. Et2O, THF) ● Reduces all carbonyl groups Ch. 12 - 18 Examples O (1) R OH O OR' O R 2. H+, H2O H 2. H+, H2O H H OH R H H + HOR' OH 1. LiAlH4, Et2O (3) R 2. H+, H2O 1. LiAlH4, Et2O (2) R OH 1. LiAlH4, Et2O R H H Ch. 12 - 19 Mechanism H O R OR' + H O Al H R H OR' H O R'O + H OH R H H H O H O H R H H R H Al H H o Esters are reduced to 1 alcohols Ch. 12 - 20 3B. Sodium Borohydride NaBH4 ● less reactive and less basic than LiAlH4 ● can use protic solvent (e.g. ROH) ● reduces only more reactive carbonyl groups (i.e. aldehydes and ketones) but not reactive towards esters or carboxylic acids Ch. 12 - 21 Examples O (1) R H O H2O R R' H2O H H OH NaBH4 (2) R OH NaBH4 R H R' Ch. 12 - 22 Mechanism O R R' O H + H B H R H OH R H R' H H O H R' Aldehydes are reduced to 1° alcohols & ketones are reduced to 2° alcohols Ch. 12 - 23 3C. Overall Summary of LiAlH4 and NaBH4 Reactivity reduced by LiAlH4 reduced by NaBH4 O O O < < R O O R OR' < R R' R H ease of reduction Ch. 12 - 24 4. Oxidation of Alcohols 4A. Oxidation of Primary Alcohols to Aldehydes R OH 1o alcohol O [O] R O [O] H aldehyde R OH carboxylic acid The oxidation of aldehydes to carboxylic acids in aqueous solutions is easier than o oxidation of 1 alcohols to aldehydes It is, therefore, difficult to stop the oxidation o of a 1 alcohol to the aldehyde stage unless specialized reagents are used Ch. 12 - 25 PCC oxidation ● Reagent PCC = N [CrO3Cl] H (Pyridinium chlorochromate) CrO3 + HCl + N Pyridine (C5H5N) N H [CrO3Cl] Pyridinium chlorochromate (PCC) Ch. 12 - 26 PCC oxidation R OH OH R R' CH2Cl2 PCC R' R CH2Cl2 R CH2Cl2 H O PCC OH R O PCC R R' No Reaction Ch. 12 - 27 4B. Oxidation of Primary Alcohols to Carboxylic Acids O - KMnO4, OH H2O, heat R H3O+ O K O R OH H2CrO4 (chromic acid) R OH Chromic acid (H2CrO4) usually prepared by [CrO3 or Na2Cr2O7] + aqueous H2SO4 Jones reagent Ch. 12 - 28 Jones oxidation ● Reagent: CrO3 + H2SO4 ● A Cr(VI) oxidant R OH OH R H2SO4 (orange solution) + R R' R' H2SO4 (orange solution) CrO3 R" H2SO4 OH Cr(III) (green) O CrO3 OH R O CrO3 + R R' Cr(III) (green) No Reaction Ch. 12 - 29 4D. Mechanism of Chromate Oxidations Formation of the Chromate Ester H H H3C H3C O C H O + HO H Cr O H3C O H O O H H O O Cr C H3C H O O O H H H H H H O + H3C O C H Cr OH H H O O H3C O O H3C H3C Cr O C H O O O H H H Ch. 12 - 30 The oxidation step O H3C O C H3C H + H H Cr OH O H3C O C O + Cr H3C + H O OH O H H O Ch. 12 - 31 4E. A Chemical Test for Primary and Secondary Alcohols R OH OH R H2SO4 (orange solution) + R R' R' H2SO4 (orange solution) CrO3 R" H2SO4 OH Cr(III) (green) O CrO3 OH R O CrO3 + R R' Cr(III) (green) No Reaction Ch. 12 - 32 4F. Spectroscopic Evidence for Alcohols Alcohols give rise to broad O-H stretching absorptions from 3200 to 3600 cm-1 in IR spectra The alcohol hydroxyl hydrogen typically produces a broad 1H NMR signal of variable chemical shift which can be eliminated by exchange with deuterium from D2O Hydrogen atoms on the carbon of a 1o or 2o alcohol produce a signal in the 1H NMR spectrum between 3.3 and 4.0 ppm that integrates for 2 and 1 hydrogens, respectively The 13C NMR spectrum of an alcohol shows a signal between 50 and 90 ppm for the alcohol carbon Ch. 12 - 33 5. Organometallic Compounds Compounds that contain carbon-metal bonds are called organometallic compounds C M primarily ionic (M = Na or K) C : M (M = Mg or Li) C M primarily covalent (M = Pb, Sn, Hg or Tl) Ch. 12 - 34 6. Preparation of Organolithium & Organomagnesium Compounds 6A. Organolithium Compounds Preparation of organolithium compounds R X + 2 Li Et2O (or THF) Order of reactivity of RX ● RI > RBr > RCl RLi + LiX Ch. 12 - 35 Example (80% - 90%) Et2O Br + 2 Li -10oC Li + LiBr Ch. 12 - 36 6B. Grignard Reagents Preparation of organomagnesium compounds (Grignard reagents) R Ar X X + + Mg Mg Et2O Et2O RMgX ArMgX Order of reactivity of RX ● RI > RBr > RCl Ch. 12 - 37 Example Br + Mg THF MgBr Ch. 12 - 38 7. Reactions of Organolithium and Organomagnesium Compounds 7A. Reactions with Compounds Containing Acidic Hydrogen Atoms RMgX ~ R:MgX RLi ~ R:Li Grignard reagents and organolithium compounds are very strong bases R MgX + H Y (or RLi) R (Y = O, N or S) H + Y + Mg2+ + X Ch. 12 - 39 Examples ● As base (1) CH3MgBr + H2O H3C H + OH + Mg2+ + Br MgBr (2) + CH3OH + CH3O + Mg2+ + Br Ch. 12 - 40 Examples ● As base (3) H + H3C MgBr MgBr + H CH3 A good method for the preparation of alkynylmagnesium halides Ch. 12 - 41 7B. Reactions of Grignard Reagents with Epoxides (Oxiranes) Grignard reagents react as nucleophiles with epoxides (oxiranes), providing convenient synthesis of alcohols RMgBr + O then H2O R OH Ch. 12 - 42 Via SN2 reaction R O R O H+, H2O R OH (1o alcohol) Ch. 12 - 43 Also work for substituted epoxides RMgBr + O H then H2O RMgBr + H (2o alcohol) R" R' OH R' R' O R then H2O R OH R" R' (3o alcohol) Ch. 12 - 44 7C. Reactions of Grignard Reagents with Carbonyl Compounds O + R R"MgX R' OH 1. Et2O 2. H3O + R R' R" R' = H (aldehyde) R' = alkyl (ketone) Ch. 12 - 45 Mechanism O + R R" O MgX R R' H OH R O MgX R" R' H H R" R' Ch. 12 - 46 8. Alcohols from Grignard Reagents O + R R"MgX R' OH 1. Et2O 2. H3O + R R' R" R' = H (aldehyde) R' = alkyl (ketone) Ch. 12 - 47 R, R’ = H (formaldehyde) o ● 1 alcohol R MgX O O MgX + R H H formaldehyde H H3O+ OH R H H H 1o alcohol Ch. 12 - 48 R = alkyl, R’ = H (higher aldehydes) o ● 2 alcohol R MgX O O MgX + R R' H higher aldehyde H H3O+ OH R R' R' H 2o alcohol Ch. 12 - 49 R, R’ = alkyl (ketone) o ● 3 alcohol R MgX O O + R R' R" ketone R' R" NH3Cl H2O OH R MgX R' R" 3o alcohol Ch. 12 - 50 Reaction with esters o ● 3 alcohol O + R OR' R"MgX OH 1. Et2O + 2. H3O R R" R" + R'OH Ch. 12 - 51 Mechanism O + R" R O MgX R OR' MgX OR' R" O R'O + R H OH R R" R" O H O H R MgX R" R" MgX R" R" Ch. 12 - 52 Examples MgBr (1) O Et2O + H OH H OMgBr H3O+ H H (1o alcohol) Ch. 12 - 53 Examples MgI (2) O Et2O + H3C H OH OMgI CH3 H3O+ CH3 H (2o alcohol) Ch. 12 - 54 Examples O (3) MgBr + Ph Ph OH (3o alcohol) Ph Et2O Ph H3O+ Ph Ph OMgBr Ch. 12 - 55 Examples O (4) MgI Et2O + Ph Ph OMe MgI OMgI O MgI OMe O Ph Ph OH H3O+ Ph (3o alcohol) Ch. 12 - 56 8A. How to Plan a Grignard Synthesis Synthesis of OH Me Me Ch. 12 - 57 Method 1 ● Retrosynthetic analysis OH MgBr Me Me O + Me Me disconnection ● Synthesis MgBr + Me O OH 1. Et2O + 2. H O 3 Me Me Me Ch. 12 - 58 Method 2 ● Retrosynthetic analysis OH Me Me MeMgBr O Me + disconnection ● Synthesis MeMgBr + O OH Me 1. Et2O 2. H3O+ Me Me Ch. 12 - 59 Method 3 ● Retrosynthetic analysis OH disconnection Me Me O OEt + 2 MeMgBr disconnection ● Synthesis O OEt OH 1. Et2O + 2. H O 3 + 2 MeMgBr Me Me Ch. 12 - 60 8B. Restrictions on the Use of Grignard Reagents Grignard reagents are useful nucleophiles but they are also very strong bases It is not possible to prepare a Grignard reagent from a compound that contains any hydrogen more acidic than the hydrogen atoms of an alkane or alkene Ch. 12 - 61 A Grignard reagent cannot be prepared from a compound containing an –OH group, an –NH– group, an –SH group, a –CO2H group, or an –SO3H group Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 62 Grignard reagents cannot be prepared in the presence of the following groups because they will react with them: OH, NH2, SO3H, SH, O O C R, NO2, C OR, N, C H, O O H, CO2H, NHR, NH2, O Ch. 12 - 63 8C. The Use of Lithium Reagents R Li + O OLi R organolithium reagent aldehyde or ketone lithium alkoxide H3O+ OH R alcohol Organolithium reagents have the advantage of being somewhat more reactive than Grignard reagents although they are more difficult to prepare and handle Ch. 12 - 64 8D. The Use of Sodium Alkynides Preparation of sodium alkynides R NaNH2 H R -NH3 Reaction via ketones (or aldehydes) O R Na ONa Na + R OH H3O+ R Ch. 12 - 65 9. Protecting Groups HO I OH How? HO Ch. 12 - 66 Retrosynthetic analysis OH O HO MgBr + HO disconnection Br HO However Br HO Mg Et2O BrMg O MgBr H O acidic proton H powerful base Ch. 12 - 67 Need to “protect” the –OH group first HO Br (protection) OH "P"O "P"O Mg, Et2O O 1. 2. H3O+ Br "P"O MgBr (no acidic OH group) (deprotection) OH HO Ch. 12 - 68 TBSCl imidazole DMF Synthesis Br HO (protection) Br TBSO Me TBSCl = tBu Si Mg, Et2O Cl Me Imidazole = N N H MgBr TBSO O DMF = H N O Me 1. Me (a polar aprotic solvent) OH HO 2. H3O+ OH Bu4N F THF (deprotection) TBSO Ch. 12 - 69 END OF CHAPTER 12 Ch. 12 - 70