Lab 1.1: Lab Skills and Techniques
advertisement

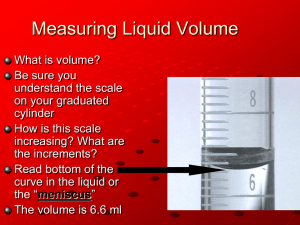
The Graduated Cylinder • Volume The Thermometer • Temperature The Electronic Balance • Mass Handling Solids/Transferring Solids to a Test Tube Decanting and Filtering a Solid The Laboratory Burner Liquids in graduated Cylinders How precisely you can measure volume depends on the size and type of graduated cylinder you use. You should be able to estimate between the marked lines…graduations. It is important to notice what each line or interval on the graduated cylinder represents. Different kinds of graduated cylinders are set up differently. A 10 milliliter cylinder, for example, usually has one tenth of a milliliter for each graduation Some have two-tenths milliliter for each graduation. The way to check this is to count the divisions between consecutive numbers. Estimate your measurements by reading between the lines, you should be able to read one digit past the marked scale. numbers are 10, 20, 30, etc., so there is a 10 milliliter increment between them. Since there are 10 divisions between consecutive numbers, each division represents one milliliter. Therefore, you should be able to estimate to tenths of a milliliter by reading between the lines. On your 50 mL cylinder each mark is one mL So read to the tenths place Just like on the 100 mL cylinder Marked to the 10ths Estimate to the 100ths Measurement has 2 places past the decimal liquids pull up the sides of a glass container This curvature is called the meniscus. Concave meniscus Read the center bottom of the meniscus. In some plastic cylinders water has a flat surface. In that case top or bottom doesn't matter, but we can still say use the center rather than the edges. some materials where the curve goes the other way. A Convex Meniscus Read the top center of the meniscus. Increase the visibility of the visibility of the meniscus by placing a paper or your hand behind it. 1. 2. 3. 4. 5. 6. Add some water to your 100 ml graduated cylinder. Read the volume of the water. Record in your lab notebook. Add some water to you 50 mL cylinder. Record the volume in your lab notebook. Repeat with your 10 mL cylinder. Add all the water to a clean beaker. Measures Temperature Use the Celsius Scale Degree is the Unit Based on freezing and boiling point of water 0 degrees C 100 degrees C do not have to be shaken down. hold a thermometer while using it. The container it is in may or may not be able to support it. All you have to do is put the thermometer in the liquid and watch the alcohol level change. When it stops changing, read the alcohol level. This is your measurement. Estimate between the lines. Mentally divide the graduations (marks) into 10 equal parts in order to read to a tenth of a degree. PARTIAL IMMERSION THERMOMETERS ARE DESIGNED TO INDICATE THE ACTUAL TEMPERATURE WHEN A PORTION OF ITS STEM IS EXPOSED TO THE TEMPERATURE BEING MEASURED. Immersion Line must be below the water level 1. Measure the temperature of the water. Make sure it’s turned on and registering zero. If not hit zero/tare to zero the balance. Do not place chemicals directly on the balance pan Do not place hot materials on the balance Do not place anything too heavy on the balance (200 grams max) Always weigh the chemicals in or on something • a weighing dish, a beaker, or a piece of folded paper. Remember to weigh the container first or adjust (tare or zero) for its mass. Be careful not to spill any chemicals on or around the balance. If you do, clean up immediately! If for some reason your balance doesn't seem to be adjusted properly, let the instructor know. Don't just try to fix it yourself. 1. 2. 3. Mass about 10 grams of NaCl (table salt) using the electronic balance. Add the solid to the beaker of water and Stir. Record your observations. Add one teaspoon of sand to the salt and water mixture. Stir. Record your observations. Decant: pour off any excess water after allowing the solid to settle Filter the sand from the salt water mixture as directed by the teacher. Weigh solid onto a piece of paper or weigh boat Push edges of paper or boat together so that they form a trough Slide solid into the test tube Remove stopper with back of hand. Hold stopper in hand while picking up the bottle and pouring the liquid down a stirring rod to prevent splashing. Do not put the stopper down. Gas Jet Barrel Base…stays cool Gas Adjustment knob Air Adjustment Clear the area of any flammable materials Close the gas adjustment completely then open 3-4 turns Adjust air if necessary Have striker ready Turn gas on at gas jet Light burner If you do not get it lit in 2 strikes… turn gas off and start over. 1. 2. Light the burner as demonstrated. Extinguish the burner. Repeat. Make sure each partner can do it efficiently. Set up a ring stand, burner, and wire gauze as demonstrated. Make 25 mL of salt water. Set the beaker of saltwater onto the wire gauze. Light the burner and put it under the wire gauze. Evaporate the water from the salt. Observe the crystals left behind. Use a hand lens. What do you notice about the crystals? Don’t look directly into a test tube being heated. Don’t point it at yourself or others. Move it back and forth through the flame to control the heating. Be careful that it does not boil out. It will heat much faster than you expect. How good are your lab skills? Illustrates the importance of carefully following lab procedure and making careful measurements in order to achieve reproducible results. You have 20 minutes. Including answering lab questions. Work quickly, but carefully. You will be able to assess your skills at the end of this activity.