Spefiic Heat - EOM Questions
advertisement

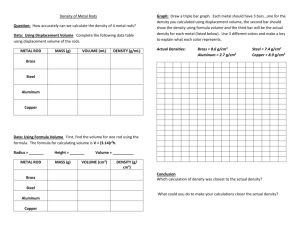
Specific Heat End of Module Questions / Concept Review Questions Remember that H = s × m × ΔT where: H = Heat transfer, s = specific heat, m = mass, and T = change in temperature. Note: this is the same equation that we used for water, but since the specific heat (s) of water = 1 Hwater = swater × mwater × ΔTwater = 1 × mwater × ΔTwater = mwater × ΔTwater Thus, for water and water only, we don’t need to include a separate term for specific heat (“s”) when calculating the heat needed to change temperature. FOR EVERYTHING ELSE, we need to ALWAYS include the specific heat (“s”). Use the definition of specific heat and the result of your experiments to answer the following questions. Use the back of this sheet or another piece of paper to show your work when applicable. 1. What units are used to express each quantity in the equation above? Quantity Units Quantity Units H (heat transfer) = s (specific heat) = m (mass) = T (change in temperature) = 2. Rearrange the equation above to show how to calculate the following: T = m= s= 1 3. When 1 cal is added to 1 g of rock and another 1 ca; is added to 1 g of an unknown metal. The temperate of the rock increases by 5 oC, while the temperature of the metal increases by 10 oC. Does the rock or the metal have a higher specific heat? Justify your answer. 4. Complete the following sentences given the following conditions. Substance #1 has a specific heat of 1.0 cal/goC. Substance #2 has specific heat of 0.5 cal/goC. Substance #3 has a specific heat of 10 cal/goC. Substance # has the highest specific heat, while substance # has the lowest specific heat. In order to change 10g of each substance by 1oC, you’ll need to add the most heat to: . Fill in blanks in the tables below: Substance Heat needed to change 1g by 1oC #1 1 cal/goC Heat needed to change 10g by 1oC Heat needed to change 10g by 10oC #2 1000 cal/goC #3 Substance what is the ΔT after 50 calories is added to 1g of each material #1 #2 #3 2 3. Examine the devise provided that contains color thermites attached to approximately equal amounts of aluminum, brass, copper, and steel (iron). Remember your previous experiments, which provide information about the specific heat of aluminum and steel. Which metal (aluminum or steel) has a higher specific heat: Which metal (aluminum or steel) should change temperature more when 1000 calories of heat is added. Thought question: Predict which metal (aluminum or steel) will change temperature faster when they are both compared to the same heat source? Explain your reasoning: Given that the specific heat of brass is lower than the specific heat of aluminum: Which metal (aluminum or brass) will change temperature more when 1000 calories of heat is added. Thought question: Which metal (aluminum or brass) will change temperature faster when they are both compared to the same heat source? Now test your predictions by placing the tips (and only the tips) of the metal in a beaker of hot water and watch how fast the temperatures of the metals change. Describe the results and compare your observations with your predictions. 3