Notes Unit 6-2
advertisement

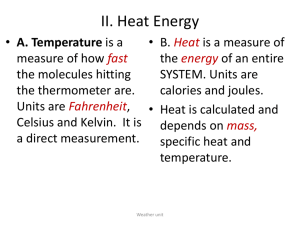
Warm Up 1-19-16 1. Write a scenario where energy is transferred (ex. making popcorn) 2. A hot frying pan is immersed in water. Describe how heat is being transfer. 3. Turning gas to liquid a _____thermic reaction, because ________ Agenda Ice Melting Activity Notes Unit 6-2 WS Specific heat Homework Finish WS Ice Melting •Place a small ice cube in a plastic beaker, glass beaker, and metal rectangle •Retry the experiment with different amount of ice •Were the temperature of the plastic, glass and metal the same? (before you put ice on them) •How fast do ice melt on each material? •Why do you think this happen? Unit 6 - 2 Specific Heat Heat vs. Temperature •Heat is energy in transit – Measured in joules (J) and calories (cal) Heat vs. Temperature Kinetic Energy: energy of an object due to the object’s motion -Temperature - the measure of the average kinetic energy of the random motion of particles in a substance – The hotter the substance, the faster the particles are moving Heat vs. Temperature • Measured in 3 different temperature scales: Fahrenheit, Celsius, and Kelvin • Kelvin is based on absolute zero scale. At zero °Kelvin, all molecules freeze & stop moving °K = °C + 273 °C = °K – 273 °C = (°F – 32) x 5⁄9 Heat vs. Temperature • Celsius Scale – 0o C - water freezes/melts – 100o C - water boils • Kelvin Scale – 0 K- molecular motion stops, also known as absolute zero – 0 Kelvin = –273o C Specific Heat • Specific heat – the amount of energy required to raise 1 gram of a substance by one degree of temperature • Water has a specific heat of 4.18 J/g·C UNIT • Transfer of heat does not always result in a temperature change (ΔT) CHECK IN Which beaker will require more heat to boil? A B CHECK IN The beaker with water and iron pan both have the same mass. Which will get hotter if the same amount of heat is applied to both? Calculating Specific Heat • Different substances respond to heat transfer differently m x ΔT x Csp = Q Symbol Unit Mass m g Change in temperature (Tfinal – Tinitial) Specific heat Heat energy ΔT K or C Csp Q J/g·C Joule Calculating Specific Heat Problems 4 gram sample of glass was heated from 274 ° K to 314 °K and required 32 J of energy. Find the specific heat of this glass m x ΔT x Csp = Q Calculating Specific Heat Problems You are boiling 100 gram of water from room temperature at 25°C to 100°C. How much heat is required? m x ΔT x Csp = Q Calculating Specific Heat Problems Determine the mass of a material its specific heat is 2 J/g·K sample absorbed 48.0 J as it was heated from 293 °K to 313 °K m x ΔT x Csp = Q Calculating Specific Heat Problems The specific heat of aluminum is 0.89 J/goC. If 435 J of energy is added to 100g of aluminum. What is the ΔT? ASSIGNMENTS • Notes Summary • Worksheet Specific Heat Answers: 1. Specific heat 2. O Kelvin 3. Endothermic 4. Exothermic 5. 4.18 6. Delta T 7. O oCelcius 8. 0.416 9. 63,536 Joules 10. 7.974 g 11. 0.2 12. 13,794 joules 13. 0.031 Answers: 14. 27, 912 joules 15. 58,050 joules 16. 12.096 grams 17. 15.01818 Celsius 18. Object B, lower specific heat means it requires less heat for temperature to increase


![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)



