Name Date Period ______ Properties of Liquids Identify the most
advertisement
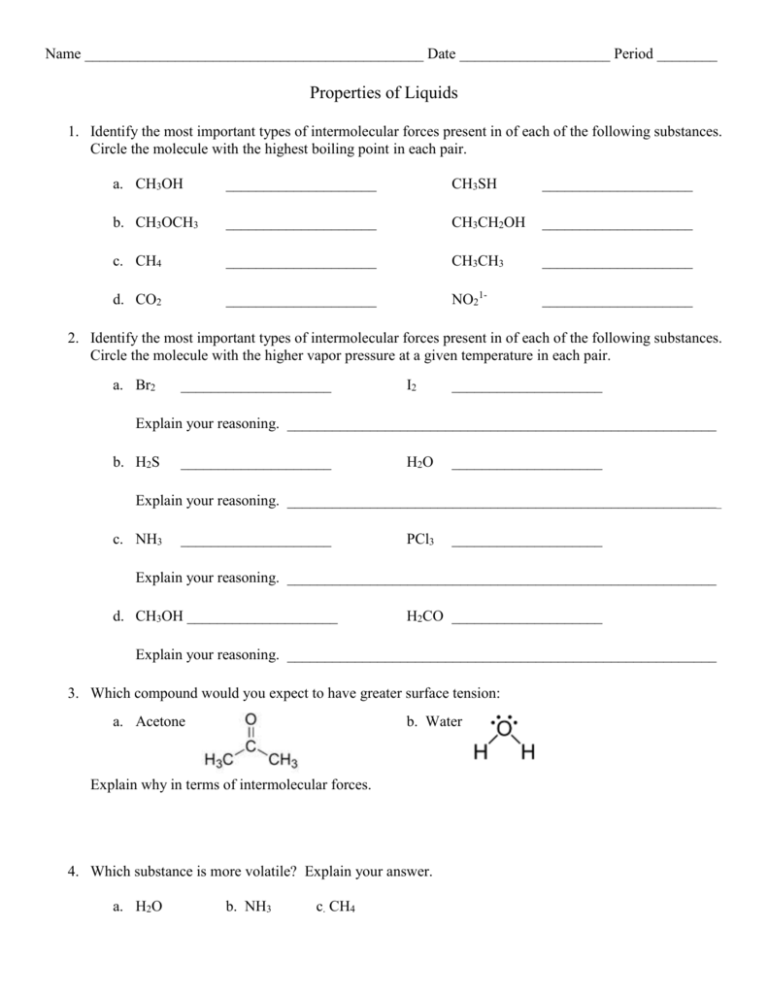
Name _____________________________________________ Date ____________________ Period ________ Properties of Liquids 1. Identify the most important types of intermolecular forces present in of each of the following substances. Circle the molecule with the highest boiling point in each pair. a. CH3OH ____________________ CH3SH ____________________ b. CH3OCH3 ____________________ CH3CH2OH ____________________ c. CH4 ____________________ CH3CH3 ____________________ d. CO2 ____________________ NO21- ____________________ 2. Identify the most important types of intermolecular forces present in of each of the following substances. Circle the molecule with the higher vapor pressure at a given temperature in each pair. a. Br2 ____________________ I2 ____________________ Explain your reasoning. _________________________________________________________ b. H2S ____________________ H2O ____________________ Explain your reasoning. __________________________________________________________ c. NH3 ____________________ PCl3 ____________________ Explain your reasoning. _________________________________________________________ d. CH3OH ____________________ H2CO ____________________ Explain your reasoning. _________________________________________________________ 3. Which compound would you expect to have greater surface tension: a. Acetone b. Water Explain why in terms of intermolecular forces. 4. Which substance is more volatile? Explain your answer. a. H2O b. NH3 c. CH4 5. You have three covalent compounds with three very different boiling points. All of the compounds have similar molar masses and relative shape. Explain how these three compounds could have very different boiling points. 6. Use the vapor pressure graph to answer the following questions. a. What is the vapor pressure of hexane at 25oC? _______________ b. At what temperature does heptane have a vapor pressure of 310 mmHg? _______________ c. What are the approximate normal boiling points of pentane, hexane and heptane? d. At a pressure of 450 mmHg and a temperature of 70oC, is each substance a liquid, a gas or a mixture of a liquid and gas? e. Which substance has the highest vapor pressure at 30oC? _______________ f. Which substance has the highest intermolecular forces? _______________ g. What type of intermolecular force is found in all of the substances? _______________