Intermolecular Forces, Liquids, and Solids
advertisement

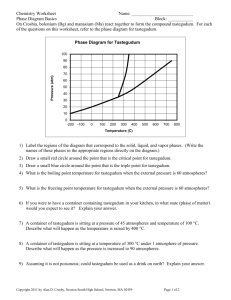
Intermolecular Forces, Liquids, and Solids Chapter 11 BLB 11th Questions? Why does water have such anomalous properties? Why do you add salt to the water before cooking pasta? How can ice melt below 0°C? How does antifreeze work? Answer: Solutions have different properties than pure solvents. 11.2 Intermolecular Forces The attractive forces between molecules or between ions and molecules Most properties of liquids and solids (and solutions) are due to the strength of the intermolecular forces present. Boiling point Melting (freezing) point ΔHvap° Types of Intermolecular Forces: In order from strongest to weakest: Ion-dipole – attraction of an ion and the partial charges of a polar molecule (hydration of ionic salts) Dipole-dipole – attraction between two polar molecules (water, HF, alcohols) London Dispersion (induced dipole) – instantaneous attraction between two nonpolar molecules due the polarizability of the electron cloud. Ion-dipole Dipole-dipole London dispersion Hydrogen “bonding” Special type of dipole-dipole interaction Occurs between a H atom attached to an electronegative atom (N, O, F) on one molecule and a lone pair on an electronegative element on another molecule. (whew!) Common in water(!) and alcohols (C–OH) Very strong collectively Hydrogen-bonding examples Between same molecule Between different molecules Protein structure (α-helix) DNA (double helix) Hydrogen-bonding between base pairs in DNA Why is water so weird? High specific heat (only NH3 higher) High ΔHfus° (only NH3 higher) Highest ΔHvap° Highest surface tension Higher boiling point than expected Ice floats on water. Answer: Strong network of hydrogen bonding Hydrogen bonding in ice Boiling Points (See Table 11.3) 11.3 Some Properties of Liquids Viscosity – resistance to flow Surface tension – a measure of the inward forces that must be overcome in order to expand the surface area of a liquid Cohesive – between molecules Adhesive – attraction between substance and a surface (glass ⇒ water) Capillary action 11.5 Vapor Pressure In an open container: liquid → gas (evaporation) In a closed container: liquid ⇌ gas (dynamic equilibrium) Vapor pressure – pressure exerted by the vapor in equilibrium with the liquid or solid in a closed container at a given temperature Vapor Pressure Vapor Pressure, cont. As T ↑, vapor pressure ↑. When vapor pressure = atmospheric pressure, boiling occurs. When pressure = 1 atm: normal boiling point Volatile substances have a higher vapor pressure. Vapor Pressure 11.6 Phase Diagrams Show graphically the relationship between the different phases of a substance. Solid, liquid, gas regions Normal melting and boiling points Triple point Critical point Slope of solid-liquid line Generic Phase Diagram H2O vs. CO2 H2O