Specific Heat Capacity & Calculations Presentation
advertisement
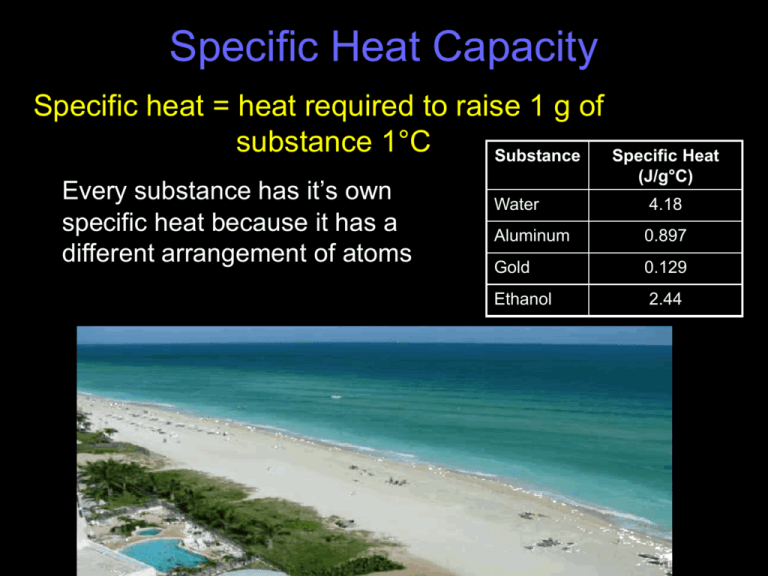
Specific Heat Capacity Specific heat = heat required to raise 1 g of substance 1°C Substance Specific Heat Every substance has it’s own specific heat because it has a different arrangement of atoms (J/g°C) Water 4.18 Aluminum 0.897 Gold 0.129 Ethanol 2.44 Specific Heat Calculations q = c × m × ΔT • • • • q = heat = J c = specific heat capacity = J / g°C m = mass = g ΔT = change in temperature =°C ΔT = Temp Final – Temp Initial Specific Heat Problems 1. If the temperature of 34.4 g of ethanol increases from 25°C to 78.8°C, how much heat has been absorbed by the ethanol? (Specific heat for ethanol = 2.44 J/(g°C) ) Specific Heat Problems 2. If the temperature of a 100 g piece of aluminum increases from 20°C to 150°C, how much heat has been absorbed by the aluminum. The specific heat of aluminum is 0.897 J/(g°C). Specific Heat Problems 3. A 4.5 g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25°C. The specific heat of gold is 0.129 J/(g°C). Specific Heat Problems 4. A 155 g sample of an unknown substance was heated from 25°C to 40°C. In the process, the substance absorbed 5696 J of energy. What is the specific heat of the substance? Specific Heat Problems 5. A 352 g sample of an unknown substance was heated from 40°C to 100°C. In the process, the substance absorbed 7696 J of energy. What is the specific heat of the substance? Specific Heat Problems 6. If the temperature of 300 g of ethanol increases from 20°C to 100°C, how much heat has been absorbed by the ethanol? (Specific heat for ethanol = 2.44 J/g°C) Specific Heat Problems 7. What is the mass of a substance that has a specific heat of 0.449 J/g°C and raises its temperature in a calorimeter from 20°C to 90°C when it release 12,300 J of energy? Specific Heat Problems 8. What is the mass of a substance that has a specific heat of 0.362 J/g°C and raises its temperature from 25°C to 110°C when it absorbs 8700 J of energy?