VLab: Density: Answers
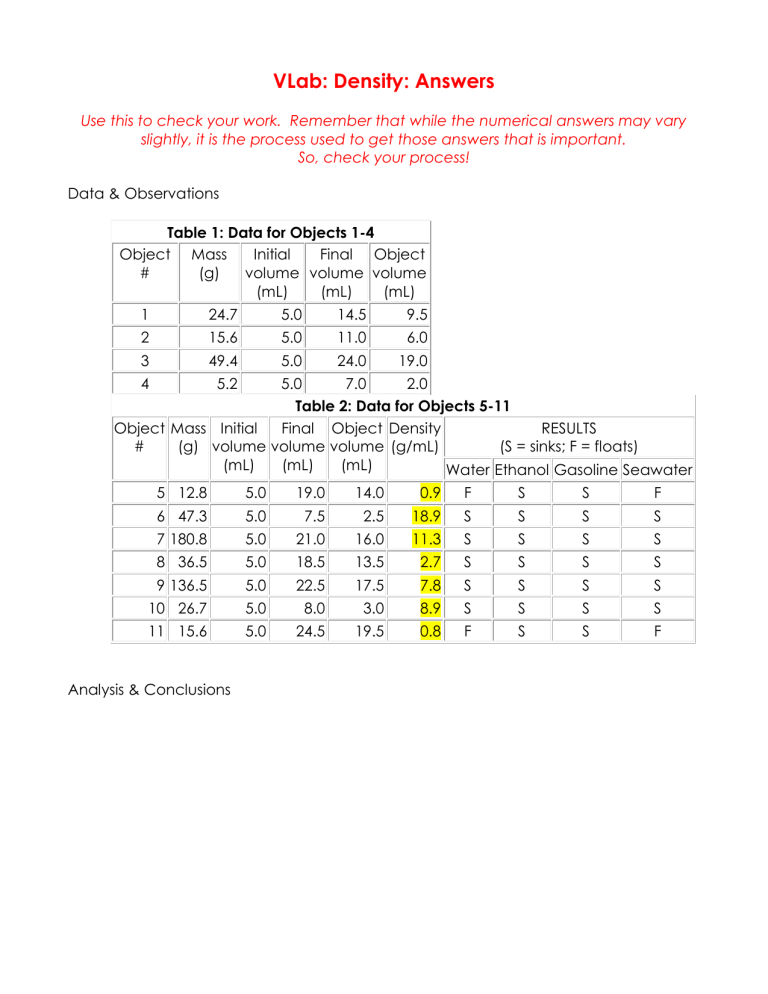
VLab: Density: Answers
Use this to check your work. Remember that while the numerical answers may vary slightly, it is the process used to get those answers that is important.
So, check your process!
Data & Observations
Table 1: Data for Objects 1-4
Object
#
Mass
(g)
Initial volume
(mL)
Final volume
(mL)
Object volume
(mL)
1
2
24.7
15.6
5.0
5.0
14.5
11.0
9.5
6.0
3
4
49.4
5.2
5.0
5.0
24.0 19.0
7.0 2.0
Table 2: Data for Objects 5-11
Object
#
Mass
(g)
Initial volume
(mL)
Final volume
(mL)
Object volume
(mL)
Density
(g/mL)
RESULTS
(S = sinks; F = floats)
Water Ethanol Gasoline Seawater
5 12.8
6 47.3
7 180.8
5.0
5.0
5.0
19.0
7.5
21.0
14.0
2.5
16.0
0.9
18.9
11.3
F
S
S
S
S
S
S
S
S
F
S
S
8 36.5
9 136.5
10 26.7
11 15.6
5.0 18.5 13.5
5.0 22.5 17.5
5.0 8.0 3.0
5.0 24.5 19.5
2.7 S
7.8 S
8.9 S
0.8 F
S
S
S
S
S
S
S
S
S
F
S
S
Analysis & Conclusions
1.
2.
slope = change in y/change in x
m = y/x
m = (39.0 - 26.0)g/(15.0 - 10.0)mL
m = 2.6 g/mL
Since mass is the y-variable and volume is the x-variable, the slope calculation requires that mass be divided by volume. Thus, the slope of the line represents the density of the glass . Also, the resulting unit for this calculation (g/mL) is appropriate for density.
3.
Since the graph of mass versus volume produces a straight line, the density of each piece of glass must be the same.
Any increase in mass is paired with the same incremental increase in volume (a linear relationship exists between the variables).
4.
Because each piece of glass has the same chemical composition, the density of the two pieces would be equivalent. Although the second piece has a greater mass, it would also have a greater volume, and the ratio of mass to volume would remain constant.
5.
Density is an intensive property. The mass of a sample has no effect upon its density.
6.
7.
#5 ice
#6 gold
#7 lead
#8 aluminum
#9 iron
#10 copper
#11 wood
The objects should separate according to their densities.
Aluminum will sink to the bottom, since it is denser than any of the liquids. Ice will sink in the ethanol, but float above the water. Wood sinks in the gasoline, but floats above the ethanol. The order of densities is indicated below: aluminum > seawater > water > ice
> ethanol > wood > gasoline
GASOLINE wood
ETHANOL ice
WATER
SEAWATER aluminum
8.
The calculated density of the crown is 12.6 g/mL (56.7 g/4.5 mL), which rounds off to 13 g/mL. This value indicates that the crown CANNOT be made of pure gold , which has a density of 19.3 g/mL. The crown could be made of lead
(density = 11.3 g/mL) and plated with a layer of gold. The resulting crown would be expected to have a density between that of lead and gold, but much closer to that of lead. A density value of 13 g/mL supports this hypothesis.
9.
d
10.
a
11.
c