Chemistry: Specific Heat Problems
advertisement

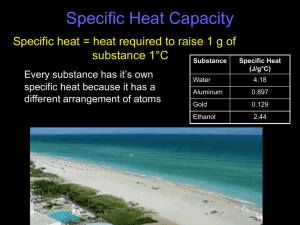
Chemistry: Specific Heat Problems Solve the following problems dealing with specific heat, cp. Use a complete set-up on each problem and show your work. 1. The specific heat of ethanol is 2.46 J/g·oC. Find the heat required to raise the temperature of 193 g of ethanol from 19oC to 35oC. 2. When a 120.0 g sample of aluminum (Al) absorbs 9612 J of energy, its temperature increases from 25oC to 115oC. Find the specific heat of aluminum. Be sure to include the correct unit for specific heat. 3. The specific heat of lead (Pb) is 0.129 J/g·oC. Find the amount of heat released when 500 g of lead are cooled from 37.2oC to 22.5oC. 4. How many kJ of energy are needed to raise the temperature of 2970 g of water from 10.55 oC to 47.32oC? Chemistry: Heat Energy Problems Solve each of the following problems. Show work and include units to earn full credit. You will need to use a reference source to find the specific heat capacity of each substance. 1. 200 g of water at 30oC are mixed with 20 g of silver at 350oC. Find the final temperature of the system. 2. 26.0 g of water at 18oC are mixed with 49.0 g of water at 70oC. Find the final temperature of the system. 3. 84.0 g of water at 22oC are mixed with 150.0 g of ethanol at 88oC. Find the final temperature of the system. 4. 24.0 g of sodium chloride at 25oC are mixed with 272 g mercury at 50oC. Find the system’s final temperature. 5. 240 g of water at 20oC are mixed with a sample of iron at 500oC. The final temperature of the system is 42oC. Find the mass of the iron sample. 6. 75.0 g of water at 30oC are mixed with 83.8 g of a solid metal at 600oC. The final temperature of the system is 50oC. What is the metal? 7. A 135 g sample of aluminum at 400oC is placed in water at 25oC. The final temperature of the system is 80oC. Find the mass of the water.