Ionic compounds WS #2
advertisement
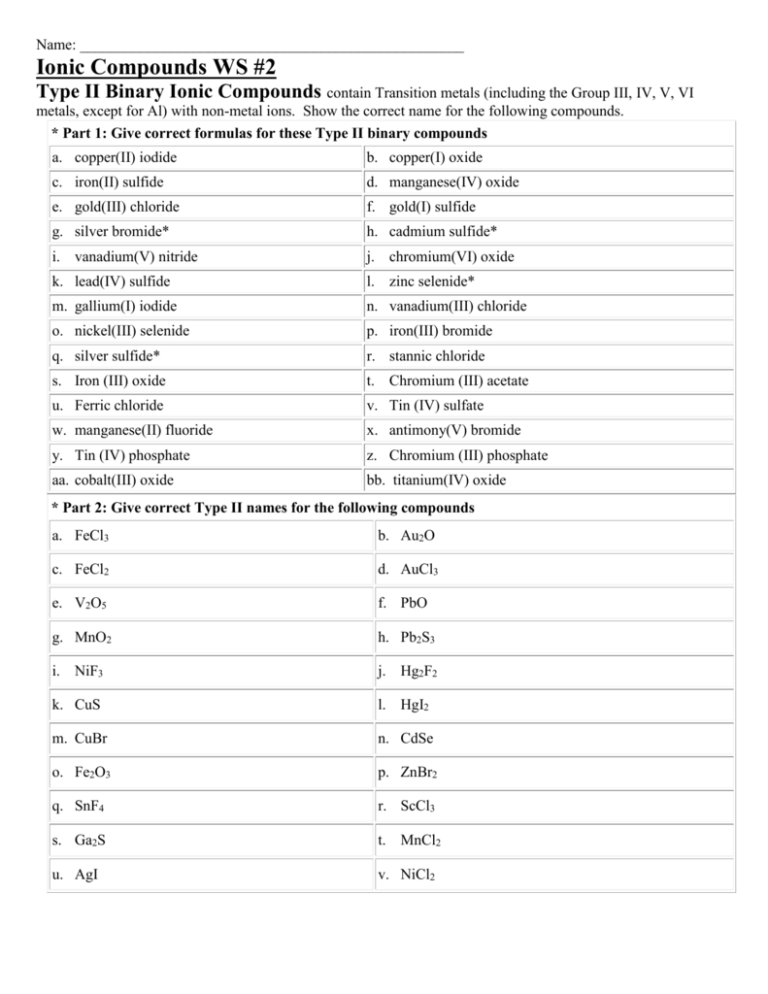
Name: ___________________________________________________ Ionic Compounds WS #2 Type II Binary Ionic Compounds contain Transition metals (including the Group III, IV, V, VI metals, except for Al) with non-metal ions. Show the correct name for the following compounds. * Part 1: Give correct formulas for these Type II binary compounds a. copper(II) iodide b. copper(I) oxide c. iron(II) sulfide d. manganese(IV) oxide e. gold(III) chloride f. gold(I) sulfide g. silver bromide* h. cadmium sulfide* i. vanadium(V) nitride j. chromium(VI) oxide k. lead(IV) sulfide l. zinc selenide* m. gallium(I) iodide n. vanadium(III) chloride o. nickel(III) selenide p. iron(III) bromide q. silver sulfide* r. stannic chloride s. Iron (III) oxide t. Chromium (III) acetate u. Ferric chloride v. Tin (IV) sulfate w. manganese(II) fluoride x. antimony(V) bromide y. Tin (IV) phosphate z. Chromium (III) phosphate aa. cobalt(III) oxide bb. titanium(IV) oxide * Part 2: Give correct Type II names for the following compounds a. FeCl3 b. Au2O c. FeCl2 d. AuCl3 e. V2O5 f. PbO g. MnO2 h. Pb2S3 i. NiF3 j. Hg2F2 k. CuS l. HgI2 m. CuBr n. CdSe o. Fe2O3 p. ZnBr2 q. SnF4 r. ScCl3 s. Ga2S t. MnCl2 u. AgI v. NiCl2 Part 3: Classical & Stock Names Directions: Determine the charge of the cation in the following ionic formulas. Write the stock name and the classical name. Check your name by writing the formula and making sure that it matches the original formula. Formula a. Cu2O Stock Name Classical Name Formula Check b. CuO c. FeCl3 d. FeCl2 e. PbO f. PbO2 g. SnCO3 h. Sn3(PO3)4 i. Pb(NO3)4 j. FePO4 k. Cu3P Part 4: Directions: Fill in the name for the formulas listed. You will need to include roman numerals for the transition metals, lead, & tin. Formula Name Formula Name a. CaSO4 l. PbCrO4 b. K2C2O4 m. CaCO3 c. Al2O3 n. BaCO3 d. SnO2 o. Cu2O e. ZnCO3 p. PbS f. RbClO4 q. Ba(OH)2 g. Pb(CH3COO)4 r. Mn(C2H3O2)2 h. CaF2 s. SnS i. AlPO4 t. HClO2 Part 5: Net Ionic Equations: Write the Net Ionic Equations for the following compounds. 1. Barium chloride 5. Calcium sulfite 2. Potassium hypochlorite 6. Nickel Nitride 3. Ammonium dichromate 7. Sodium oxalate 4. Aluminum permanganate 8. Tin (IV) dichromate











