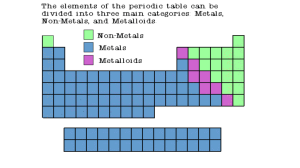
Families of the Elements Questions
advertisement

DO NOT WRITE ON! DO NOT WRITE ON! Families of the Elements Questions. Answer each question completely on a separate sheet of paper. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Give the group number, period number for the following elements: a. Cl e. Au b. Cu f. Mt c. Rn g. C d. H h. As How are period number and energy levels related? What are valence electrons? What the 2 magic numbers each element is trying to reach? If hydrogen is not an alkali metal, why do you think hydrogen was placed in this group? Why do you think the alkali metals are not found freely in nature? Why do you think the alkaline earth metals are called earth metals? Why is tungsten used in light bulbs? Why are the halogens the most reactive non metals? Explain why noble gases are unreactive. Why are elements 58-71 called lanthanoids? Why are elements 90-103 called actinoids? Which group on the periodic table has mainly radioactive elements? DO NOT WRITE ON! Write out the electron configuration for each of the following elements. Do not use the noble gas shortcut and circle the electrons that would be valence. a. K b. Hg c. Eu d. Kr e. O 14. Draw the orbital notation for the following elements. Do not use the noble gas shortcut! a. Fe b. N c. Ir d. Li 15. Now use the valance electrons trick and identify the number of valence electrons for each of the following elements: a. Ac g. Xe b. S h. Sn c. Ni i. B d. Sr j. He e. Fr k. Be f. Al l. Pt 13. DO NOT WRITE ON! DO NOT WRITE ON! 13. Write out the electron configuration for each of the following elements. Do not use the noble gas shortcut and circle the electrons that would be valence. a. K b. Hg c. Eu d. Kr e. O 14. Draw the orbital notation for the following elements. Do not use the noble gas shortcut! a. Fe b. N c. Ir d. Li 15. Now use the valance electrons trick and identify the number of valence electrons for each of the following elements: a. Ac g. Xe b. S h. Sn c. Ni i. B d. Sr j. He e. Fr k. Be f. Al l. Pt DO NOT WRITE ON! Families of the Elements Questions. Answer each question completely on a separate sheet of paper. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. DO NOT WRITE ON! Give the group number, period number for the following elements: a. Cl e. Au b. Cu f. Mt c. Rn g. C d. H h. As How are period number and energy levels related? What are valence electrons? What the 2 magic numbers each element is trying to reach? If hydrogen is not an alkali metal, why do you think hydrogen was placed in this group? Why do you think the alkali metals are not found freely in nature? Why do you think the alkaline earth metals are called earth metals? Why is tungsten used in light bulbs? Why are the halogens the most reactive non metals? Explain why noble gases are unreactive. Why are elements 58-71 called lanthanoids? Why are elements 90-103 called actinoids? Which group on the periodic table has mainly radioactive elements? DO NOT WRITE ON!